User login
Prostate Cancer in Male Seniors Part 2: Treatment
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
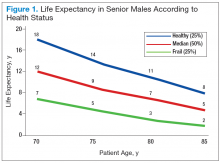
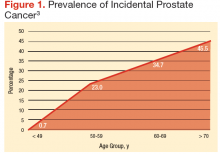
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
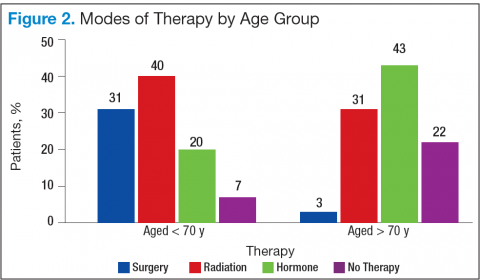
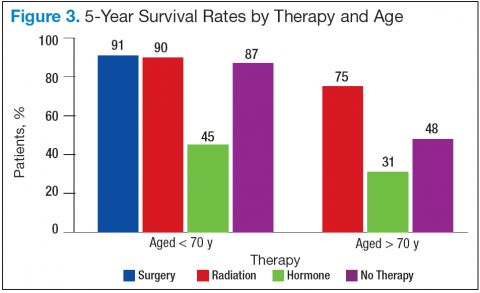
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
This article (part 2 of 2) focuses on the treatment of prostate cancer in seniors. Part 1 provided an overview of prostate cancer epidemiology, pathology, and screening in senior patients.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Recently, the International Society of Geriatric Oncology assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
Patient Evaluation
The practice guidelines of the National Comprehensive Cancer Network (NCCN) recommend using life expectancy in determining treatment.1 Prostate cancer is considered to be an indolent disease, and active therapy may be more harmful than beneficial to older patients whose life expectancy is limited because of treatment-related sequelae. Therefore, an accurate estimation of life expectancy is important in devising treatment strategies for older patients.
Age should not be the only factor in determining the life expectancy of patients, because life expectancy varies widely based on the patient’s health status, including preexisting comorbidities. Chronologic life expectancy can be found in the Social Security Administration’s life tables. Individual life expectancy is then projected by adding 50% to or deducting 50% from the chronologic life expectancy for men in the highest and lowest quartile of health, respectively. The life expectancy from the life tables can be applied with no addition or subtraction for men in the middle 2 quartiles of health status (Figure 1).2
Geriatric Assessment
Despite the increasing incidence of prostate cancer in older adults, no particular guidelines for its management exist. Compared with younger patients, older
patients with prostate cancer need to weigh the benefits of treatment vs the risks to avoid any potential adverse treatment-related quality of life (QOL) decreases. Clearly, for some patients there are no significant benefits from treatment (eg, improved survival).
The Comprehensive Geriatric Assessment has been created to properly assess aging in correlation to individual and patient-centered biologic and clinical metrics.
Following extensive literature reviews, the International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group recognized that the most important prognostic factors in evaluating health status in elderly patients with prostate cancer include comorbidities, functional dependence, and nutrition status.3 An important prognosticator of survival in prostate cancer is preexisting comorbidities. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) is considered the best metric currently available in assessing a patient’s death risk unrelated to cancer.
Another important factor influencing survival of older patients with prostate cancer is the patient’s level of independent activity. Independent functioning is evaluated
using (1) activities of daily living (ADL); and (2) the instrumental activities of daily living (IADL).4-6
Health Status Subgroups
The SIOG recommendation for prostate cancer treatment in older patients is based on a complete assessment of existing comorbidities of patients using the CIRS-G,
IADL, and ADL scales as well as the nutritional status of each patient.3,7-9 Based on these prognostic tools, the SIOG classifies the health status of elderly patients with prostate cancer into 4 prognostic health status categories: healthy, vulnerable, frail, and terminal.10,11
Patient Characteristics
Older patients generally present with highrisk prostate cancer at diagnosis.12,13 However, older patients are less likely to be treated with curative intent, resulting in
lower overall and disease-specific survivals. Nearly 40% of deaths due to prostate cancer occur in patients aged ≥ 75 years and 31% in the group aged ≥ 85 years.12,14 Recent reports have demonstrated that curative radiotherapy or surgery improved survival outcomes as well as QOL in the elderly, comparable with those seen in younger patients.
Bechis and colleagues analyzed the relationship between survival of older patients with high-risk cancer and curative local therapy.15 Treatment modalities included radical prostatectomy, external-beam radiation therapy (EBRT), watchful waiting/active surveillance, and other modalities, including primary androgen deprivation therapy (ADT). The findings were: (1) older patients more frequently presented with high-risk disease as age increased; (2) therapeutic approaches varied but were based mainly on age at diagnosis rather than on cancer risk factors (Figures 2 and 3); and (3) ADT was used more frequently in older patients compared with its use in younger patients, irrespective of the risk score, including patients with high-risk disease.
Older patients were less likely to receive radical therapy, especially surgical treatment, regardless of risk category. Forty-four percent of patients aged > 70 years with high-risk disease died of any cause at a median 5.7 years, and 21% died of prostate cancer; whereas 47% of patients aged > 75 years with high-risk disease died at a median of 5.3 years, and 20% of those died of prostate cancer.
When older patients with high-risk disease received curative local therapy, however, the mortality rate decreased.
In a study by Sun and colleagues, 4,561 senior patients who received radical prostatectomy therapy were classified into 3 age groups (aged < 60 years, aged 60 to 70 years, and aged > 70 years) based on the year of surgery (before or after 2000). Therapy outcomes were compared among the 3 groups.16 The researchers found that seniors aged > 70 years who presented with high-risk disease had poorer therapeutic outcomes. A diagnosis of advancedstage cancer and a Gleason score > 7 were more often made in patients aged > 70 years vs that of their younger counterparts. They also found greater risk of failures for these patients in biochemical recurrence, distant metastasis, and disease-specific survivals.
Most clinicians typically ruled out active treatment based on chronologic age alone, without considering existing comorbidities and overall life expectancy. According
to a study by Daskivich and colleagues, only 16% of patients aged > 75 years were aggressively treated, whereas 84% of patients aged < 55 years received aggressive curative therapy, using radical prostatectomy, radiation therapy, or brachytherapy.17
Therapeutic Approaches
Current NCCN guidelines recommend active surveillance as an option for men with low- and intermediate-risk disease with a < 10-year life expectancy and the only option for men with a < 20-year life expectancy and a very low-risk of prostate cancer (stage T1c, Gleason ≤ 6, prostate specific antigen [PSA] < 10 ng/mL, < 3 positive cores, < 50% core involvement, and PSA density < 0.15 ng/mL2). Patients who are older and have significant comorbidities should be managed with active surveillance rather than with active treatment. In the practice setting, however, studies indicated that a substantial number of older men with limited life expectancy still received aggressive treatment for low-risk cancer. Active treatment tended to decrease with age but was still common among men aged > 80 years: 25% received active local therapy, 36% received primary ADT, and only 39% received no active treatment.18
Surgery
Surgical treatment is an active therapeutic option for some patients with localized disease. Mortalities were reduced using prostatectomy vs watchful waiting, including disease-specific mortality and rates of metastasis. As newer techniques develop, laparoscopic prostatectomy may be able to provide excellent therapeutic
outcomes with quick surgical recovery times and possibly less postoperative nerve damage. Compared with younger patients, older patients experienced comparable
outcomes after surgical therapy.19-21 Despite encouraging surgical outcomes, however, surgery is not generally offered to patients aged > 70 years because
of the presumed high risks related to possible surgical complications.
Radiation Therapies
External-beam radiation therapy has been a well-established, standard mode of radiotherapy for the past several decades, among various radiation modalities, including brachytherapy (high- and low-dose radioactive seed implant therapy), cyber-knife therapy, and proton therapy. If indicated, EBRT rather than surgery is generally suggested as an active treatment for patients with localized prostate cancer. In general, EBRT and radical prostatectomy are comparable in survival
outcomes, but EBRT is preferred for older patients because it is noninvasive.21,22 Conventional EBRT technique has gradually progressed over the past several decades, advancing to 3D conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image-guided radiotherapy, and then most recently to RapidArc radiation therapy.
RapidArc radiotherapy is an advanced form of IMRT that increases dose conformity and significantly shortens daily treatment times. In contrast to the static conventional IMRT technique (requiring repeated stops to deliver radiation through a 360° rotation of the therapy machine around the patient), RapidArc radiotherapy continues to deliver radiation therapy to the targeted tumor lesion with no interruption while the therapy machine is rotating around the patient. Accordingly, radiation therapy time is much shorter (up to 8 times faster) compared with conventional IMRT radiotherapy.
Systemic Therapy
Androgen-deprivation therapy can slow cancer growth, as it inhibits androgen production, blocks androgen action, or both. For localized prostate cancers with intermittent- and high-risk for recurrence, radiation therapy combined with ADT (eg, leuprolide, goserelin, triptorelin) reduces mortality of patients compared with ADT alone. In addition, hormone therapy is used for advanced, recurrent, or metastatic prostate cancers.
Most advanced and roughly one-fifth of biologically recurrent cancers ultimately convert to castrationresistant prostate cancer and may potentially benefit from nonhormonal systemic chemotherapy. Docetaxel with or without prednisone is the agent of choice for castration-resistant symptomatic metastatic prostate cancer. Cabazitaxel is a secondgeneration taxane and approved for castration-resistantmetastatic prostate cancer. Other systemic drugs (hormonal) for chemotherapy-naïve, metastatic castration-resistant prostate cancer are abiraterone (androgen synthesis inhibitor) and enzalutamide (anti-androgen).
Low-Risk Prostate Cancers
Active surveillance would be a reasonable management option for older patients with low-risk, localized prostate cancer and limited life expectancy of < 10 years. Albertsen and colleagues reported that patients with welldifferentiated prostate cancer and limited life expectancy have little chance of death due to prostate cancer but are more likely die of other causes, such as preexisting comorbidities.23 Bill-Axelson and colleagues reported a very similar cancer-specific mortality rate of only 2.5% for patients with well-differentiated prostate cancer who are receiving either active therapy or active surveillance.24 In another study, Krakowsky and colleagues reported a 97% 10-year cancer-specific survival rate in 450 patients with a median age of 70 years, and in a randomized study, Holmberg and colleagues reported no differences in overall survival for patients aged > 65 years who were randomized to surgery or watchful waiting for early-stage prostate cancer.25,26
The literature consistently reports cancer-specific survival rates approaching 100% for patients with low-risk prostate cancer. The main concern regarding aggressive
therapy for older patients with low-risk cancer and significant comorbidities, as well as limited life expectancy, is the real possibility of overtreatment and the resultant high risk of treatment-related complications and loss in QOL. For example, surgery can lead to varying degrees of incontinence, and radiation can lead to rectal bleeding from proctitis, both severely impacting patients’ QOL.
High-Risk Prostate Cancers
Older patients with high-risk prostate cancer generally do not receive curative therapy. Bechis and colleagues examined the influence of age on disease-specific mortality.15 They found that patients aged > 75 years were more likely to be diagnosed with high-risk prostate cancer and treated with conservative therapy, such as ADT or watchful waiting, often resulting in death. They also found that the choice of therapy in older patients was based primarily on age rather than on comorbidities or other disease factors. Trends for such undertreatment were most evident in healthy seniors with high-risk cancer. The undertreatment of older patients with lower comorbidities contributes to the higher disease-specific mortality seen in the elderly population. Such healthy older patients were often overlooked solely because of their age and might have been denied the opportunity to receive curative and life-saving therapy early.
Summary
Most prostate cancers develop in older patients, and nearly one-fourth of prostate cancers are diagnosed in patients who are aged > 75 years. In addition, older patients show a higher tendency to present with highrisk prostate cancer. Furthermore, older patients have a higher risk of death compared with that of younger
patients, although many of them still die of causes other than prostate cancer. The most important prognostic factors in older patients, as recognized by the SIOG Prostate Cancer Working Group, included comorbidities, dependence status, and nutrition status. Management decisions for older patients with prostate cancer should be individualized and formulated based on remaining life expectancy, the patient’s functional performance and health status, as well as coexisting comorbidities and patient-specific prognostic characteristics of the prostate cancer, such as stage, Gleason score, and PSA values.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations— including indications, contraindications, warnings, and adverse effects—before administering pharmacologic
therapy to patients.
Click here to read the digital edition.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network Website http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated October 24, 2014. Accessed June 14, 2015.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73(1):68-91.
4. Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453-471.
5. Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513-1519.
6. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
7. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914-919.
8. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205-206.
9. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186.
10. Droz JP, Balducci L, Bolla M, et al. Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010;106(4):462-469.
11. Fitzpatrick JM, Graefen M, Payne HA, Scotté F, Aapro MS. A Comment on the International Society of Geriatric Oncology guidelines: evidence-based advice for the clinical setting. Oncologist. 2012;17(suppl 1):31-35.
12. Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Sem Radiat Oncol. 2012;22(4):284-294.
13. Howlader N, Noon AM, Krapcho M, et al. SEER cancer statistics review 1975-2008. SEER Website. http://seer.cancer.gov/archive/csr/1975_2008. Updated November 10, 2011. Accessed June 10, 2015.
14. Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280-1283.
15. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29(2):235-241.
16. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
17. Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058-2066.
18. Cooperburg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(1):2141-2149.
19. Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101(5):541-546.
20. Siddiqui SA, Sengupta S, Slezak JM, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175(3 pt 1):952-957.
21. Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Semin Radiat Oncol. 2013;23(3):198-205.
22. Payne HA, Hughes S. Radical radiotherapy for high-risk prostate cancer in older men. Oncologist. 2012;17(suppl 1):9-15.
23. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095-2101.
24. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
25. Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131-135.
26. Holmberg L, Bill-Axelson A, Helgesen F, et al; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New Engl J Med. 2002;347(11):781-789.
Role of Radiosurgery in the Treatment of Brain Metastasis
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
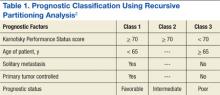
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
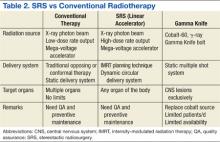
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
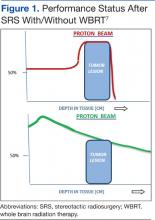
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
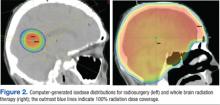
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
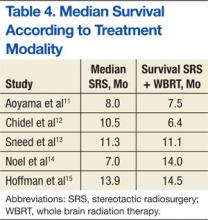
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
Prostate Cancer in Seniors, Part 1: Epidemiology, Pathology, and Screening
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostate cancer is the most common cancer and the second leading cause of cancer deaths in men. The incidence of prostate cancer continues to rise. Roughly 220,800 men were expected to be newly diagnosed with prostate cancer in 2015.1 As the population ages and overall life expectancy increases, prostate cancer is likely to become a growing health care burden, especially because prostate cancer is primarily a disease of elderly males.
There have been no specific practice guidelines for managing prostate cancer in older adults, and the current management of older patients with prostate cancer is often suboptimal. Fortunately, the International Society of Geriatric Oncology recently assembled a multidisciplinary prostate cancer working group, which has begun offering guidelines on evidence-based treatments of prostate cancer in the geriatric population.
This article (part 1 of 2) provides a brief overview of prostate cancer epidemiology, pathology and screening in senior patients. The second part (to be published in August 2015) will focus on treatment.
Epidemiology
Currently more than 2 million men are estimated to have prostate cancer in the U.S. About 18% to 20% of U.S. males have a lifetime risk of developing prostate cancer. Prostate cancer is mainly a disease of seniors aged between 60 and 70 years—the median age of prostate cancer at diagnosis is about 65 to 68 years. About 65% of new prostate cancers are diagnosed in males aged 65 years and 25% in males > aged 75 years.2 Most older patients with prostate cancer do not die of prostate cancer.
As the life expectancy of the general population increases, the risk of developing prostate cancer among seniors is also expected to proportionally rise. Historically, the cancer-specific mortality rate of prostate cancer in patients aged > 70 years was only 29% if managed either with active surveillance or hormonal manipulation.
Prevalence of Incidental Prostate Cancer
There is an abrupt age-dependent increase of prostate cancer incidence from the 5th decade of life on. Furthermore, there is a 1 in 3 chance of incidental prostate cancer in men aged between 60 to 69 years and a 46% prevalence in men aged > 70 years. Yin and colleagues found that 12% of patients in their study group harbored incidental, preclinical prostate cancer.3-5 The increasing prostate cancer incidence showed a strong and clear correlation with advancing age (Figure 1).
The lifetime probability of being diagnosed with prostate cancer also increases significantly with age.6,7 Patients with a life expectancy of < 5 years are unlikely to benefit from cancer screening and may be more likely to experience complications and potential treatment-related harm as a result of screening. Therefore, estimating the patient’s residual life expectancy is a critical factor in the decision-making process for patients with prostate cancer. Life expectancy can differ, depending on various factors besides age, such as health, functional status, and medical comorbidities. The estimated age-related life expectancy for seniors has gradually increased over the previous 5 decades.8
Risk Factors
There are several risk factors for prostate cancer: age, race, and ethnicity; genetic factors; environmental and socioeconomic status; dietary status; and others. However, these factors may play only a limited role in the risk of prostate cancer, and a cautious approach and careful interpretation are required for their application in clinical practice.9,10
- Age. There is a sudden and dramatic increase in the prevalence of prostate cancer with advancing age. Prostate cancer is rarely diagnosed in men aged < 40 years, but thereafter, the incidence of prostate cancer climbs steadily.11 Surprisingly, subclinical microscopic prostate cancer was found at autopsy (death from unrelated causes) in a majority of senior males in their eighth decade of life.3
- Race/ethnicity. Epidemiologic studies in the U.S. found the highest incidence of prostate cancer in African American men (incidence rate of 235 per 100,000 African American vs 150 per 100,000 white men). Also, African American men tended to present with higher grades and stages of prostate cancer. There were much lower incidence rates of prostate cancer in Asian Americans and Pacific Islanders, Hispanics, and American Indian and Alaska Natives (90 per 100,000, 126 per 100,000, and 78 per 100,000, respectively).9,10
- Diet. According to researchers, the western diet may be an important risk factors for prostate cancer. However, the actual relationship between obesity and prostate cancer is somewhat unclear, and any correlation is at present highly controversial. Some investigators have postulated that obesity can contribute to the development of prostate cancer; other studies have clearly established that obese patients, once diagnosed with prostate cancer, have inferior outcomes irrespective of the treatment modality used. Other studies, however, have suggested that certain hormonal profiles related to obesity may be protective against the development of prostate cancer.12,13
Pathologic Evaluation
The original Gleason Grading System was devised based on the careful analysis of the cellular pattern of tumor architecture, using a 5-point scale: Tumor cells similar to normal-appearing prostate tissue were designated Gleason 1, 2, and 3; whereas cells/glands appearing abnormal were
designated Gleason 4 and 5. The total Gleason score is the sum of the 2 most representative patterns, applied to both prostatectomy and needle biopsy specimens. The main differences from the original Gleason system, proposed by the 2005 International Society of Urological Pathology Modified Gleason System, are summarized in Table 1.
Early Detection and Screening
Although prostate cancer screening with prostate-specific antigen (PSA) detects many prostate cancer cases, concerns surrounding universal screening include the potential for overdiagnosis and overtreatment, along with the real possibility for adverse effects and complications from treatment. In addition, the recommendations for prostate cancer screening are not consistent among the various national health organizations. The American Cancer Society (ACS) recommends having an informed discussion between the health care provider and patient about the possible benefits and harms of screening. The discussion should not be initiated in men aged < 50 years (or aged < 45 years in men with high-risk features), and there is no need for screening in men with a life expectancy of < 10 years.
Prostate cancer screening may detect cancers that would not have become clinically significant. This is even more likely to be true when life expectancy decreases. Informed screening decisions in senior adults should be made according to the individual’s values and preferences in addition to the estimated outcomes and possible harms as a result of screening. The National Comprehensive Cancer Network offers similar recommendation to the ACS Screening Guidelines (Table 2).
Screening Recommendations for Seniors
There have been no generally recognized guidelines on prostate cancer screening for seniors, although recently, Konety and colleagues published “The Iowa Prostate Cancer Consensus” for elderly prostate cancer patients (Table 3).17 The consensus includes:
- More prostate cancers are detected at an earlier stage, but many of them would never become clinically apparent in most patients’ life times
- A reduced mortality (either overall or disease specific) from screening is not proven during the course of 10-year follow-up
- Harms related to diagnostic and therapeutic procedures develop early and remain for an extended period, causing a negative impact on quality of life
- The small benefits of screening leading up to a prostatectomy are seen only after 12 years of follow-up and may be limited to a certain population group of patients who are aged < 65 years
- Current recommendations discourage the routine screening of seniors with short life expectancies (< 10 years) and depend on existing comorbidities and disease group risk
Conclusion
Prostate cancer is the most common cancer in American men and the second most common cause of cancer death. Prostate cancer is almost twice as common among African Americans vs whites, and much less common in Asian Americans and Pacific Islanders, Hispanics, American Indian and Alaska Natives. Prostate cancer is generally a cancer of older seniors, and nearly 80% of seniors are estimated to harbor subclinical prostate cancer by their eighth decade of life.8 Prostate cancer screening is not universally recommended, and major professional associations support an informed, evidence-based, shared decision-making process between medical professionals and patients. This decision should include the careful consideration of patients’ life expectancy and existing medical comorbidities, always weighing the potential benefits against the possible screening and treatment-related harms.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16-22.
3. Yin M, Bastacky S, Chandran U, Becich MJ, Dhir R. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179(3):892-895.
4. Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B.. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Euro Urol. 2005;48(5):739-744.
5. Sánchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54(3):238-247.
6. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96.
7. Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182(5):2242-2248.
8. Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47(5):273-287.
9. Miocinovic R. Epidemiology and risk factors. In: Klein EA, Jones JP, eds. Management of Prostate Cancer. 3rd ed. Totowa, NJ: Humana Press; 2013:1-11.
10. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3-12.
11. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
12. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):557-563.
13. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118-1126.
14. Epstein JI. An update of the Gleason grading system. J Urol. 2010;183(2):433-440.
15. Egevad L, Mazzucchelli R, Montironi R. Implications of the International Society of Urological Pathology modified Gleason grading system. Arch Pathol Lab Med. 2012;136(4):426-434.
16. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostate Carcinoma. Am J Surg Pathol. 2005;29(9): 1228-1242.
17. Konety BR, Sharp VJ, Raut H, Williams RD. Screening and management of prostate cancer in elderly men: the Iowa Prostate Cancer Consensus. Urology. 2008;71(3):511-514.