User login
Role of Radiosurgery in the Treatment of Brain Metastasis
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
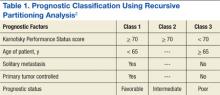
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
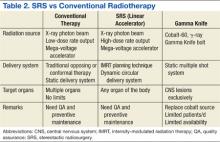
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
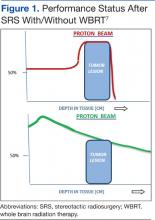
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
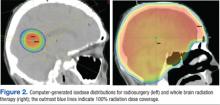
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
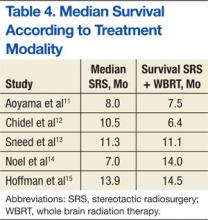
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Since the 1980s, patients with a single intracranial metastatic lesion traditionally have been treated with surgery followed by whole brain radiation therapy (WBRT). However, there is growing concern about the debilitating cognitive effects associated with WBRT in long-term survivors.
Limbrick and colleagues studied the outcomes of surgery followed by stereotactic radiosurgery (SRS) instead of WBRT and found that the less invasive surgical resection (SR) followed by SRS was an equally effective therapeutic option for the treatment of patients with limited metastatic disease to the brain.1 Median overall survival (OS) was 20 months and was 22 and 13 months for Classes 1 and 2 recursive partitioning analysis (RPA) patients, respectively. Recursive partitioning analysis refers to 3 prognostic classes based on a database of 3 trial studies and 1,200 patients (Table 1).2 According to RPA, the best survival was observed in Class 1 patients, and the worst survival was seen in Class 3 patients. Limbrick and colleagues found that survival outcome was equivalent to or greater than that reported by other studies using surgery plus WBRT or SRS plus WBRT.1 The WBRT was not used and was reserved as salvage therapy in cases of initial failure such as disease progression of brain metastasis.
Radiation Therapies
Stereotactic radiosurgery is not a surgical procedure but a newly developed radiotherapy technique. It is a highly precise, intensive form of radiation therapy, focused on the tumor, with the goal of protecting the surrounding normal brain tissue as much as possible. Radiosurgery was initially introduced with the Gamma Knife by Lars Leksell several decades ago in order to deliver an intense radiation dose to a small, well-defined, single focal point using extreme precision. Stereotactic radiosurgery delivers efficient and focused radiation treatment to the tumor lesion.
There are 2 practical and commercially available radiation delivery systems for SRS: linear accelerator (LINAC)-based radiosurgery and Gamma Knife systems. Use of the Gamma Knife is limited largely to treatment of central nervous system (CNS) malignancies and certain head and neck cancers. Linear accelerator-based SRS is applicable to neoplasms in any organ system of the body (Table 2).
Proton therapy is yet another evolving and completely different mode of radiation therapy. There are currently 14 proton therapy centers in operation in the U.S., and 11 more centers are now under construction. Proton therapy uses charged heavy-particle therapy using proton beams, whereas conventional LINAC-based radiotherapy is X-ray radiotherapy, which uses high energy photon beams. Because of their relatively large mass, protons have little scatter of radiation to surrounding normal structures and can remain sharply focused on the tumor lesion. Accordingly, proton therapy delivers negligible radiation doses beyond tumor lesions, and much of the surrounding normal tissues can be saved from excessive and unnecessary radiation doses.
Related: Bone Metastasis: A Concise Overview
A single proton beam produces a narrow Bragg peak dose distribution at depth, and multiple consecutive stepwise series of different energies of proton beams are needed to administer complete coverage of the target tumor volume. The accumulation of these beam energies produces a uniform radiation dose distribution covering the entire tumor volume (Figure 1). In spite of the theoretical beneficial effects of proton beam therapy, more clinical experience is needed for it to be validated. Even then, the significantly higher costs of proton therapy represent another barrier to its wider implementation. Proton beam radiosurgery is still, in large part, an evolving technology, not widely and uniformly available.
Role of Radiosurgery
Photon (X-ray)-based radiosurgery can be an alternative to craniotomy. Patients can return to their activities immediately after treatment. The ideal candidate for radiosurgery should have a small tumor (1-3 cm is best) with a well-defined margin. Retrospective studies reported no significant difference in therapy outcomes between the 2 therapies.3,4 Additional benefits of radiosurgery include low morbidity and mortality. Furthermore, radiosurgery can be applied to tumors near critical structures, such as the thalamus, basal ganglia, and brainstem, that are otherwise surgically inaccessible.
Most brain metastases are well defined and spherical, so they are ideally treated using SRS (Figure 1). Additionally, the brain is encased in the bony skull, which prevents significant intrafraction motion and provides a reproducible fidulial for accurate setup. Radiosurgery can tailor the radiation dose in order to precisely concentrate radiation distribution to the tumor lesion with a rapid dose falloff beyond the margin of the tumor bed, so surrounding normal brain tissues are spared from high-dose radiation. In sharp contrast, WBRT indiscriminately irradiates the entire brain without sparing the adjacent normal brain tissue (Figure 2). However, because of its limited dose distribution, radiosurgery offers no protection elsewhere in the brain from future metastasis, which is a benefit of whole brain radiation.
Future Use of SBRT
Based on successful experience with intracranial lesions, stereotactic techniques have been expanded to additional anatomical body sites other than the brain. Stereotactic body radiation therapy (SBRT), also called stereotactic body ablative radiotherapy, is progressively gaining acceptance and is being applied to various extracranial tumors, especially lung cancers and hepatic malignancies. Dosimetric studies and early phase clinical trials have clearly established the feasibility, safety, and efficacy of SBRT for certain tumor sites, such as lung, liver, kidney, spine, and paraspinal tumors. Additionally, SBRT may reduce treatment time and therapy costs and thus provide increased convenience to patients.
Effectiveness of SRS
Stafinski and colleagues conducted a meta-analysis of randomized trials to study the effectiveness of SRS in improving the survival as well as the quality of life (QOL) and functional status following SRS of patients with brain metastasis.5 This study found that SRS plus WBRT increased OS for patients with single brain metastasis compared with WBRT alone. Although no significant difference in OS was found in patients with multiple brain metastases, the addition of SRS to WBRT improved the local control and functional independence of this group of patients.
Related: Palliative Radiotherapy for the Management of Metastatic Cancer
Kondziolka and colleagues reported a local failure rate at 1 year of merely 8% following SRS boost therapy after WBRT compared with 100% without SRS.6 There was also a remarkable difference in median time to local failure—36 months vs 6 months, respectively. A randomized study designed to assess the possible benefit of SRS for the treatment of brain metastasis found a survival gain for patients with a single brain metastasis with a median survival time of 6.5 months (SRS) vs 4.9 months (no SRS).7
There are sparse data and reporting related to QOL measurements after SRS for brain metastasis. Andrews and colleagues reported improved functional and independent abilities at 6 months after completion of SRS therapy.7 The criteria used in that study for performance assessments included the Karnofsky Performance Status (KPS) scale, the need for steroid use, and mental status. They found that KPS improvement was statistically significant, and patients were able to decrease the dosage of steroid medication at 6 months after therapy with SRS (Table 3). Despite these reports suggesting superior outcomes with SRS, more rigorous investigations that adequately control for other factors influencing QOL in patients with cancer are needed.
Two major limitations of SRS include large tumor size and multiple numbers of metastatic brain lesions. As the radiation dose to adjacent normal brain tissue quickly increases with larger tumor lesions (> 3-4 cm), the complication risks consequently rise proportionally, necessitating a decrease in the prescribed dose. Patients with poor performance status (< 70 KPS) and presence of active/progressive extracranial disease are also not ideal candidates for SRS.
Other unfavorable conditions for SRS include life expectancy of < 6 months, metastatic lesions in the posterior fossa, and severe acute CNS symptoms due to increased intracranial pressure, brain edema, or massive tumor effects. These factors do not necessarily contraindicate SRS but can increase the risks of such treatment. The authors recommend an experienced multispecialty approach to patients presenting with these findings.
Managing Brain Metastastis
To prevent symptoms related to brain edema (due to brain tumor itself and/or radiation-induced edema), steroid medication is generally administered to most patients, 1 to 3 days prior to initiation of radiation therapy. Corticosteroid use typically results in rapid improvement of existing CNS symptoms, such as headaches, and helps prevent the development of additional CNS symptoms due to radiation therapy-induced cerebral edema. A dexamethasone dose as low as 4 mg per day may be effective for prophylaxis if no symptoms or signs of increased intracranial pressure or altered consciousness exist. If the patient experiences symptomatic elevations in intracranial pressure, however, a 16-mg dose of dexamethasone per day orally, following a loading dose of 10-mg IV dexamethasone, should be considered. The latter scenario is not common.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
The benefits of steroids, however, need to be carefully balanced against the possible adverse effects (AEs) associated with steroid use, including peripheral edema, gastrointestinal bleeding, risk of infections, hyperglycemia, insomnia, as well as mental status changes, such as anxiety, depression, and confusion. In long-term users, the additional AEs of oral candidiasis and osteoporosis should also be taken into account.
Craniotomy vs SRS
A retrospective study by Schöggl and colleagues compared single brain metastasis cases treated using either Gamma Knife or brain surgery followed by WBRT (30 Gy/10 fractions).3 Local control was significantly better after radiosurgery (95% vs 83%), and median survival was 12 months and 9 months after radiosurgery and brain surgery, respectively. There was no significant difference in OS.
Another comparative study of SR and SRS for solitary brain metastasis revealed no statistically significant difference in survival between the 2 therapeutic modalities (SR or SRS); the 1-year survival rate was 62% (SR) and 56% (SRS).4 A significant prognostic factor for survival was a good performance status of the patients. There was, however, a significant difference in local tumor control: None of the patients in the SRS group experienced local recurrence in contrast to 19 (58%) patients in the SR group.
Whereas selection criteria and treatment choice depend to a large extent on tumor location, tumor size, and availability of SRS, most studies demonstrated that both surgery and SRS result in comparable OS rates for patients with a single brain metastasis.
Multiple Brain Metastases
Jawahar and colleagues studied the role of SRS for multiple brain metastases.8 In their retrospective review of 50 patients with ≥ 3 brain metastases, they found an overall response rate (RR) of 82% and a median survival of 12 months after SRS. As a result of similar studies and their own data, Hasegawa and colleagues recommended radiosurgery alone as initial therapy for patients with a limited number of brain metastases.9
SRS vs SRS Plus WBRT
Studies on the role of SRS plus WBRT are somewhat conflicting. A Radiation Therapy Oncology Group study revealed statistically significant improvement in median survival when SRS boost therapy was added to WBRT in patients with a single brain metastasis compared with SRS alone.5 According to another study, the addition of SRS to WBRT provided better intracranial and local control of metastatic tumors.10
A randomized controlled study by Aoyama and colleagues reported no survival improvement using SRS and WBRT in patients with 1 to 4 brain metastases compared with SRS alone.11 In addition, a retrospective review found no difference in median survival outcomes between SRS alone and SRS plus WBRT (Table 4). In the absence of a clear survival benefit with the use of both modalities and in light of the added toxicity of WBRT, most clinicians have ceased offering both modalities upfront and instead reserve WBRT as a salvage option in cases of subsequent intracranial progression of disease.
SRS vs WBRT
In general, both SR (crainotomy) and SRS for the treatment of brain metastases seem to be effective therapeutic modalities. Comparisons of both treatments did not reveal significant differences and showed similar outcomes after treatment of smaller lesions. For example, Rades and colleagues reported that SRS alone is as effective as surgery and WBRT for limited metastatic lesions (< 2) in the brain.16 Either SRS or surgery can be used, depending on performance status and metastatic burden (size, location, number of lesions, etc).
There are some inconsistencies in the final results of various studies, such as survival, local tumor control, mortality rate, and pattern of failures. For large, symptomatic brain metastasis, initial surgical debulking remains the preferred approach as a way of achieving immediate decompression and relief of swelling/symptoms. Additionally, for patients who have > 10 brain lesions and/or a histology that corroborates diffuse subclinical involvement of the brain parenchyma (eg, small-cell lung cancer), WBRT is also typically preferred to upfront SRS. Alternatively, radiosurgery is the preferred method for fewer and smaller lesions as a way of minimizing the toxicity from whole brain irradiation. The optimal treatment of multiple small brain metastases remains controversial with some investigators recommending SRS for > 4 metastases only in the setting of controlled extracranial disease based on the more favorable expected survival of such patients.
Multidisciplinary Approach for Lung and Breast Cancers
Prognostic outcomes of patients with brain metastases can vary by primary cancer type. Therefore, clinicians should consider cancer-specific management and tailor their recommendation for specific types of radiation depending on the individual cancer diagnosis. Various investigators have attempted to develop disease-specific prognostic tools to aid clinicians in their decision making. For example, Sperduto and colleagues analyzed significant indexes and diagnosis-specific prognostic factors and published the diagnostic-specific graded prognostic assessment factors.17 They were able to identify several significant prognostic factors, specific to different primary cancer types.
Bimodality Therapies
For certain cancers such as lung and breast primary cancers, bimodality therapy using chemotherapy and radiation treatment should be considered based on promising responses reported in the literature.
Recent studies on the efficacy of chemotherapy for brain metastases from small-cell lung cancer (43%-82%) have also been reported.18-20 Postmus and colleagues reported superior RR of 57% with combination chemotherapy and radiation vs a 22% RR for chemotherapy alone.21 They also found favorable long-term survival trends in patients treated with combined radiochemotherapy.
The efficacy of chemotherapy in non-small cell carcinoma of the lung has been reported in multiple phase 2 studies using various chemotherapeutic agents. The reported RR ranged from 35% to 50%.22-24 Comparative studies of combined chemoradiotherapy demonstrated a 33% RR in contrast to a 27% RR for combined therapy or chemotherapy alone, respectively. However, no difference was noted in median survival rates.25
Care must be taken when interpreting these studies due to heterogeneity of the patient population studied and a lack of data on potential synergistic toxicities between radiation to the CNS and systemic therapy. The authors generally avoid concurrent chemotherapy during CNS irradiation in patients who may have significant survival times > 1 year.
The prognosis of breast cancer patients with brain metastasis largely depends on the number and size of metastatic brain lesions, performance status, extracranial or systemic involvement, and systemic treatment following brain irradiation. The median survival of patients with brain metastasis and radiation therapy is generally about 18 months. The median survival for patients with breast cancer who develop brain metastasis was 3 years from diagnosis of the primary breast cancer.26
Recent advances in systemic agents/options for patients with breast cancer can significantly affect the decision-making process in regard to the treatment of brain lesions in these patients. For example, a few retrospective studies have clearly demonstrated the beneficial effect of trastuzumab in patients with breast cancer with brain metastasis. The median OS in HER2-positive patients with brain metastasis was significantly extended to 41 months when treated with HER2-targeted trastuzumab vs only 13 months for patients who received no treatment.27,28 As a result of the expected prolonged survival, SRS for small and isolated brain lesions has recently become a much more attractive option as a way of mitigating the deleterious long-term effect of whole brain irradiation (memory decline, somnolence, etc).
Summary
Stereotactic radiosurgery is a newly developed radiation therapy technique of highly conformal and focused radiation. For the treatment of patients with favorable prognostic factors and limited brain metastases, especially single brain metastasis, crainiotomy and SRS seems similarly effective and appropriate choices of therapy. Some studies question the possible benefits of additional WBRT to local therapy, such as crainiotomy or radiosurgery.
Some authors recommend deferral of WBRT after local brain therapy and reserving it for salvage therapy in cases of recurrence or progression of brain disease because of possible long-term AEs of whole brain irradiation as well as deterioration of QOL in long-term survivors. Thus, the role of additional WBRT to other local therapy has not been fully settled; further randomized studies may be necessary. Due to the controversies and complexities surrounding the treatment choices for patients with brain disease, all treatment decisions should be individualized and should involve close multidisciplinary collaboration between neurosurgeons, medical oncologists, and radiation oncologists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.
1. Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71(3):280-288.
2. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
3. Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien). 2000;142(6):621-626.
4. O’Neill BP, Iturria NJ, Link MJ, Pollock BE, Ballman KV, O’Fallon JR. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55(5):1169-1176.
5. Stafinski T, Jhangri GS, Yan E, Manon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32(3):203-213.
6. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427-434.
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
8. Jawahar A, Shaya M, Campbell P, et al. Role of stereotactic radiosurgery as a primary treatment option in the management of newly diagnosed multiple (3-6) intracranial metastases. Surg Neurol. 2005;64(3):207-212.
9. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery. 2003;52(6):1318-1326.
10. Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and whole brain radiotherapy (WBRT) plus a stereotactic boost (WBRT+SRS) for one to three brain metastases. Strahlenther Onkol. 2008;184(12):655-662.
11. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483-2491.
12. Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
13. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
14. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41(3):333-343.
15. Hoffman R, Sneed PK, McDermott MW, et al. Radiosurgery for brain metastases from primary lung carcinoma. Cancer J. 2001;7(2):121-131.
16. Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109(12):2515-2521.
17. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
18. Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61(1):147-150.
19. Tanaka H, Takifuj N, Masuda N, et al. [Systemic chemotherapy for brain metastases from small-cell lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(4):492-497. Japanese.
20. Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7(7):216-222.
21. Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organisation for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18(19):3400-3408.
22. Cortes J, Rodriguez J, Aramendia JM, et al. Frontline paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64(1):28-35.
23. Minotti V, Crinò L, Meacci ML, et al. Chemotherapy with cisplatin and teniposide for cerebral metastases in non-small cell lung cancer. Lung Cancer. 1998;20(2):23-28.
24. Fujita A, Fukuoka S, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patient with brain metastases from non-small cell lung cancer. Oncology. 2000;59(4):291-295.
25. Robinet G, Thomas R, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12(1):29-67.
26. Kiricuta IC, Kölbl O, Willner J, Bohndorf W. Central nervous system metastases in breast cancer. J Cancer Res Clin Oncol. 1992;118(7):542-546.
27. Berghoff AS, Bago-Horvath Z, Dubsky P, et al. Impact of HER-2-targeted therapy on overall survival in patients with HER-2 positive metastatic breast cancer. Breast J. 2013;19(2):149-155.
28. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Truastzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56-62.