User login
For which patients is maternal oxygen supplementation of value?
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P
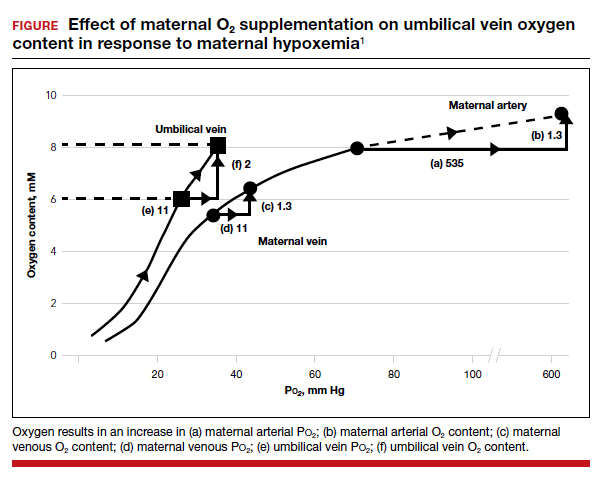
Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P

Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
Raghuraman N, Temming LA, Doering MM, et al. Maternal oxygen supplementation compared with room air for intrauterine resuscitation: a systematic review and meta-analysis. JAMA Pediatr. January 4, 2021. doi:10.1001/jamapediatrics.2020.5351.
EXPERT COMMENTARY
Maternal oxygen supplementation is widely used in labor for nonreassuring fetal heart rate (FHR) tracings, although its efficacy is uncertain for preventing fetal acidosis, operative intervention, or sequelae of neonatal encephalopathy. Recently, Raghuraman and colleagues reported the results of a systematic review and meta-analysis that included 16 randomized controlled trials. A total of 1,078 women were included in the oxygen group and 974 in the room air group. The primary outcome was umbilical artery pH; 14 trials reported on this outcome.
After analyzing the pooled and stratified results of the effect of maternal oxygen supplementation versus room air on umbilical artery gas measures, the authors concluded that peripartum oxygen supplementation is not associated with clinically relevant improvement in umbilical artery pH. They acknowledged, however, that the published studies were heterogeneous, lacked data on the association of oxygen supplementation with neonatal outcome, and did not assess oxygen use for abnormal FHR tracings, except for one trial with category II FHR tracings.
Effects of O2 supplementation
As maternal arterial hemoglobin is nearly saturated under normal conditions, maternal hyperoxia produces only modest increases in umbilical vein P

Fetal hypoxemia and acidosis can result from an interruption or an impairment of the mother-to-placenta-to-fetus oxygen pathway. With some interruptions of the oxygen pathway, such as placental abruption and complete cord occlusion–induced bradycardia, there would be less impact of maternal hyperoxia. By contrast, with other oxygen pathway impairments, such as reduced oxygen transfer with placental insufficiency, maternal hyperoxia can be of greater value by increasing maternal uterine artery and vein P
Continue to: Circumstances that may benefit from O2 supplementation...
Circumstances that may benefit from O2 supplementation
Late FHR decelerations reflect impairment of oxygen transfer and thus represent the heart rate pattern that is most likely to benefit from maternal hyperoxia. However, recurrent late decelerations occur in less than 2% of low-risk patients in labor,3 and severe levels of acidosis (umbilical artery pH <7.0 or base deficit [BD] ≥12 mmol/L) occur in only 1% to 2% of near-term or term deliveries.4,5
Variable decelerations also reflect fetal hypoxia and are much more common than late decelerations, so they also may benefit from O2 supplementation. Regardless, O2 supplementation should be seen only as a temporizing strategy while other resuscitative actions are initiated, including preparation for operative delivery, if indicated.
In a prior study by Raghuraman and colleagues (1 of only 4 studies that met selection criteria of oxygen supplementation for patients in labor), newborns of patients not receiving oxygen demonstrated 95% confidence limits of umbilical artery pH (7.24–7.28) and BD (2.9–4.3) well within the normal range.6 Thus, the low prevalence of cases in which a benefit might be anticipated and the low incidence of severe acidosis challenges the design of prospective studies to detect statistically and clinically significant changes in blood gas measures and newborn outcomes.
The normal mild fetal acidosis that develops during labor is likely a result of recurrent interruption of uterine placental blood flow during uterine contractions7 and is unlikely to benefit from maternal hyperoxia. Similarly, as placental oxygen transfer is predominantly flow rather than diffusion limited,8 oxygen supplementation is unlikely to improve severe variable FHR decelerations. Thus, a randomized study of hyperoxia in unselected laboring patients is unlikely to have a measurable effect on clinically significant acidosis.
Oxygen transport pathway guides treatment
For the present, an understanding of oxygen transport can guide clinical oxygen use. Thus, mothers with relative hypoxemia will unquestionably benefit with supplemental oxygen administration. Similarly, fetuses at risk for placental dysfunction (for example, growth restriction, postterm) and particularly those manifesting evidence of impaired oxygen transport (that is, late decelerations) may be most likely to benefit from the increased O2 gradient. For patients with reduced maternal uterine perfusion (such as hypotension or hypovolemia), pressors and/or fluid volume are likely to be more effective, while amnioinfusion is of greater value for umbilical cord compression patterns. A reduction in uterine activity may be of benefit to all fetuses exhibiting compromise. Due to the modest impact on fetal oxygen content, maternal hyperoxia does not produce significant fetal oxidative stress as measured by fetal malondialdehyde levels.
In view of the lack of demonstrated adverse effects of maternal supplemental oxygen, clinicians should not hesitate to use it. However, clinicians should recognize that supplemental oxygen is likely to be of value only in patients with significant impairment in the oxygen pathway, and they should choose additional intrauterine resuscitative measures focused on the etiology.
MICHAEL G. ROSS, MD, MPH,
AND BRYAN S. RICHARDSON, MD
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.
- McNanley T, Woods J. Placental physiology. Glob Libr Women’s Med. (ISSN: 1756-2228). 2008. doi: 10.3843 /GLOWM.10195.
- Richardson BS. Fetal adaptive responses to asphyxia. Clin Perinatol. 1989;16:595-611.
- Sameshima H, Ikenoue T. Predictive value of late decelerations of fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19-23.
- Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824-831.
- Kelly R, Ramaiah SM, Sheridan H, et al. Dose-dependent relationship between acidosis at birth and likelihood of death or cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2018;103:F567-F572.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172:818-823.
- Ramsey EM, Corner JW Jr, Donner MW. Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213-225.
- Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596:5523-5534.