User login
Transdermal rivastigmine for dementia
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms
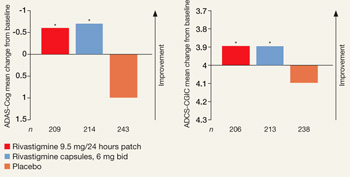
*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
The rivastigmine patch is the first transdermal treatment for symptoms of mild to moderate Alzheimer’s disease (AD) and mild to moderate Parkinson’s disease dementia (Table). Rivastigmine, a cholinesterase inhibitor, is the only therapy approved for both indications.
Table
Rivastigmine transdermal patch: Fast facts
| Brand name: Exelon Patch |
| Class: Cholinesterase inhibitor |
| Indication: Symptomatic treatment of mild to moderate Alzheimer’s-type dementia and mild to moderate dementia associated with Parkinson’s disease |
| Manufacturer: Novartis Pharmaceuticals, Inc. |
| Dosing forms: 4.6 and 9.5 mg/24 hours transdermal patches (5 cm2 and 10 cm2, respectively) |
| Recommended dosage: Start with 4.6 mg/24 hours patch for ≥4 weeks, followed by a one-step increase to the target dose 9.5 mg/24 hours patch* |
| *Unless the patient is taking oral rivastigmine (see ‘Transitioning to rivastigmine patch,’) |
Clinical implications
The rivastigmine patch offers continuous drug delivery through the skin into the bloodstream over 24 hours.1 This may reduce the incidence of side effects compared with oral rivastigmine,2 making optimal therapeutic doses easier to attain.3 The target dose 9.5 mg/24 hours patch provides efficacy similar to the highest recommended rivastigmine capsule dose (6 mg bid for a total of 12 mg/d).2
How it works
The rivastigmine patch uses matrix technology, which enables delivery of a large amount of drug from a small surface area.4 The patch is available in 2 dosage forms:
- a 5-cm2 size containing 9 mg of rivastigmine that delivers 4.6 mg/24 hours
- a 10-cm2 size containing 18 mg of rivastigmine that delivers 9.5 mg/24 hours.
Each patch consists of 4 layers: the backing layer, an acrylic drug matrix, a silicone adhesive matrix, and an overlapping release liner that is removed and discarded before the patch is applied.1
Cholinesterase inhibitors are believed to exert their effects by increasing available levels of the neurotransmitter acetylcholine in the brain. Two studies have demonstrated that cognitive improvements associated with rivastigmine treatment correlate significantly with cholinesterase inhibition.5,6 In 1 study, rivastigmine’s inhibitory effects on cholinesterase were sustained for 12 months.6
Pharmacokinetics
Rivastigmine is metabolized by its target cholinesterase enzymes to the decarbamylated metabolite NAP 226-90, which has minimal acetylcholinesterase inhibition and is excreted through the urine.1 As a result of its low accumulation potential and cytochrome P 450-independent metabolism, rivastigmine has low potential for pharmacokinetic drug–drug interactions. This lack of interaction has been confirmed for many drugs commonly taken by elderly patients, such as digoxin, nonsteroidal anti-inflammatory drugs, and estrogens.7
Rivastigmine has a half-life of 1 to 2 hours, so it is rapidly cleared.8 In the event of a serious reaction, significant clearance of rivastigmine from the body would occur within 3 hours of patch removal.
Centrally mediated cholinergic gastrointestinal (GI) side effects associated with oral rivastigmine are related to high maximum plasma concentrations (Cmax) and short time interval to Cmax (Tmax).9 In an open-label, parallel-group study of 51 AD patients that compared rivastigmine patches with rivastigmine capsules, transdermal administration was associated with slower increases to lower peak plasma concentrations (prolonged Tmax and reduced Cmax), and less fluctuation in plasma concentration.1 Despite these effects, the rivastigmine 9.5 mg/24 hours patch provided drug exposure comparable to the highest dose of capsules (6 mg bid for a total of 12 mg/d), with improved GI tolerability.3
Efficacy
Rivastigmine patch efficacy was evaluated in a single, 24-week, international, randomized, double-blind trial of 1,195 patients with AD.2 The study group represented typical patients with mild to moderate AD—age 50 to 85 years with Mini-Mental State Examination scores of 10 to 20 at baseline. Patients were randomly assigned to receive:
- 17.4 mg/24 hours rivastigmine patch (20-cm2 patch; n=303)
- 9.5 mg/24 hours rivastigmine patch (10-cm2 patch; n=293)
- 6 mg bid rivastigmine capsules (n=297)
- or placebo (n=302).
Data for the 17.4 mg/24 hours patch are not discussed here because this dose exceeds the FDA-approved maximum dosage (9.5 mg/24 hours) and is not available.
Patients in the 9.5 mg/24 hours patch group received a 4.6 mg/24 hours patch (5 cm2) for weeks 1 through 4, and then the 9.5 mg/24 hours patch for the remainder of the study. Patients in the capsule group started on 3 mg/d (1.5 mg bid) and were titrated every 4 weeks in steps of 3 mg/d to a maximum of 12 mg/d administered as 6 mg bid.
Primary outcomes were measured as mean change in score from baseline to endpoint on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Alzheimer’s Disease Co-operative Study–Clinical Global Impression of Change (ADCS-CGIC). By study endpoint, the 9.5 mg/24 hours patch and capsules, 12 mg/d, showed comparable efficacy (Figure).2 Compared with those receiving placebo, patients in the 9.5 mg/24 hours patch and capsule groups showed significant improvements in dementia symptoms, including:
- cognition
- global performance
- attention
- activities of daily living.2
Based on my clinical experience, these improvements reflect small but clinically meaningful changes that are noted by patients and caregivers.
Figure
Efficacy of transdermal rivastigmine for Alzheimer’s symptoms

*P<0.05 vs placebo
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADCS-CGIC: Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change
Source: Adapted from reference 2
In a 24-week study, transdermal rivastigmine, 9.5 mg/24 hours, and the highest recommended dose of oral rivastigmine (6 mg bid) showed comparable efficacy as measured by mean change in score on scales commonly used in Alzheimer’s disease clinical trials. ADAS-Cog assesses orientation, memory, language, praxis, and visuospatial functions. ADCS-CGIC provides a single global rating of change from baseline based on interviews with the patient and caregiver.
Safety and tolerability
Adverse events associated with rivastigmine are predominantly cholinergic; GI side effects—nausea, vomiting, and diarrhea—are observed most frequently.2 These events occur less frequently with the patch than with capsules. In the efficacy trial, patients in the 9.5 mg/24 hours rivastigmine patch group had one-third as many reports of nausea (7.2% vs 23.1%) and vomiting (6.2% vs 17.0%) compared with the 6 mg bid capsule group.2
Diarrhea was reported by 6% of subjects receiving the 9.5 mg/24 hours patch, 5% of those taking 6-mg capsule bid, and 3% receiving placebo. Fewer subjects in the 9.5 mg/24 hours patch group (3%) experienced decreased weight compared with those in the capsule group (5%). The rate of decreased weight with placebo was 1%.
Dizziness affected 2% of those in the 9.5 mg/24 hours patch and placebo groups; incidence in the capsule group was significantly higher at 8%. Headache was similar with the 9.5 mg/24 hours patch (3%) and placebo (2%), with the capsule significantly higher at 6%.2
The proportion of patients who experienced no, slight, or mild skin irritation ranged from 90% to 98%.2 The most commonly reported moderate or severe skin irritations were erythema (8% rivastigmine patch vs 4% placebo) and pruritus (7% rivastigmine patch vs 3% placebo). Two percent of patients using active patch discontinued the trial because of skin irritation.
Rivastigmine appears not to produce adverse effects on cardiac function as assessed by ECG. In clinical trials of 2,791 patients, pooled 12-lead ECG data comparing oral rivastigmine and placebo groups did not differ significantly in heart rate or PR, QRS, and QTc intervals.10
Dosing
The rivastigmine patch is administered once daily, and the recommended maintenance dose is the 9.5 mg/24 hours patch. Start patients on a 4.6 mg/24 hours patch for at least 4 weeks and then increase to the 9.5 mg/24 hours target dose if the lower dose is well tolerated.
Dosage adjustment of rivastigmine is not necessary in patients with hepatic or renal disease because of minimal liver metabolism and the acetylcholinesterase-mediated hydrolysis of rivastigmine to the inactive decarbamylated metabolite NAP 226-90, which is excreted in the urine.11
Instruct patients or caregivers to apply the patch to clean, dry, hairless skin that is free of cuts, rashes, or irritation on the upper or lower back or upper arm or chest.1 The patch has shown good adhesive properties over 24 hours, remaining attached in a range of situations, including bathing and hot weather.2 In the 9.5 mg/24 hours group of the efficacy study, 96% of patches remained attached or had slight lifting of the edges (1,336 total patch evaluations).
Transitioning to rivastigmine patch
The efficacy study included an open-label extension, during which blinding was maintained. This provided information on patients beginning rivastigmine patch therapy directly from placebo2 or transitioning from rivastigmine capsules to the target dose 9.5 mg/24 hours patch.12 Based on these results, transition patients as follows:
- Patients taking oral rivastigmine, <6 mg/d: Switch to a 4.6 mg/24 hours patch for ≥4 weeks before increasing to a 9.5 mg/24 hours patch.
- Patients taking oral rivastigmine, 6 to 12 mg/d: Switch directly to a 9.5 mg/24 hours patch.
Apply the first patch the day after the last oral dose.
Related resource
- Rivastigmine transdermal system prescribing information. www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf.
Drug brand names
- Digoxin • Lanoxin
- Rivastigmine • Exelon
- Rivastigmine transdermal
- system • Exelon Patch
Disclosure
Dr. Sadowsky is a consultant to and speaker for Forest Pharmaceuticals and Novartis Pharmaceuticals.
Acknowledgment
The author thanks Christina Mackins, PhD, a medical writer for Alpha-Plus Medical Communications Ltd, for her editorial assistance with this article. Funding for her work was provided by Novartis Pharmaceuticals.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.
1. Lefèvre G, Sedek G, Jhee S, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. J Clin Pharmacol 2007;47:471-8.
2. Winblad B, Cummings J, Andreasen N, et al. A six-month, double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease—rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456-67.
3. Oertel W, Ross JS, Eggert K, Adler G. Rationale for transdermal drug administration in Alzheimer disease. Neurology 2007;69(suppl 1):S4-S9.
4. Petersen TA. Transdermal drug formulations and process development. Pharmaceut Technol 2003;(suppl):18-21.
5. Giacobini E, Spiegel R, Enz A, et al. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 2002;109:1053-65.
6. Darreh-Shori T, Almkvist O, Guan ZZ, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 2002;59:563-72.
7. Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000;15(3):242-7.
8. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998;20:634-47.
9. Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719-39.
10. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002;42:558-68.
11. Exelon patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2007.
12. Frölich L, Barone P, Förstl H, et al. IDEAL: A 28-week open-label extension of a 24-week double-blind study of the first transdermal patch in Alzheimer’s disease. Poster presented at: 11th Congress of the European Federation of Neurological Societies; August 25-28, 2007; Brussels, Belgium.
Dr. Sadowsky is associate clinical professor of neurology, Nova Southeastern University, Fort Lauderdale, FL, and director, Premier Research Institute, Palm Beach Neurology, West Palm Beach, FL.