User login
Actinomycetoma: An Update on Diagnosis and Treatment
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
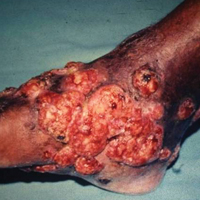
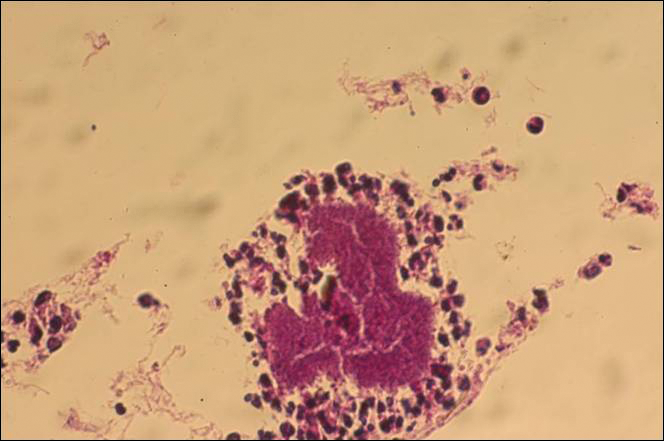
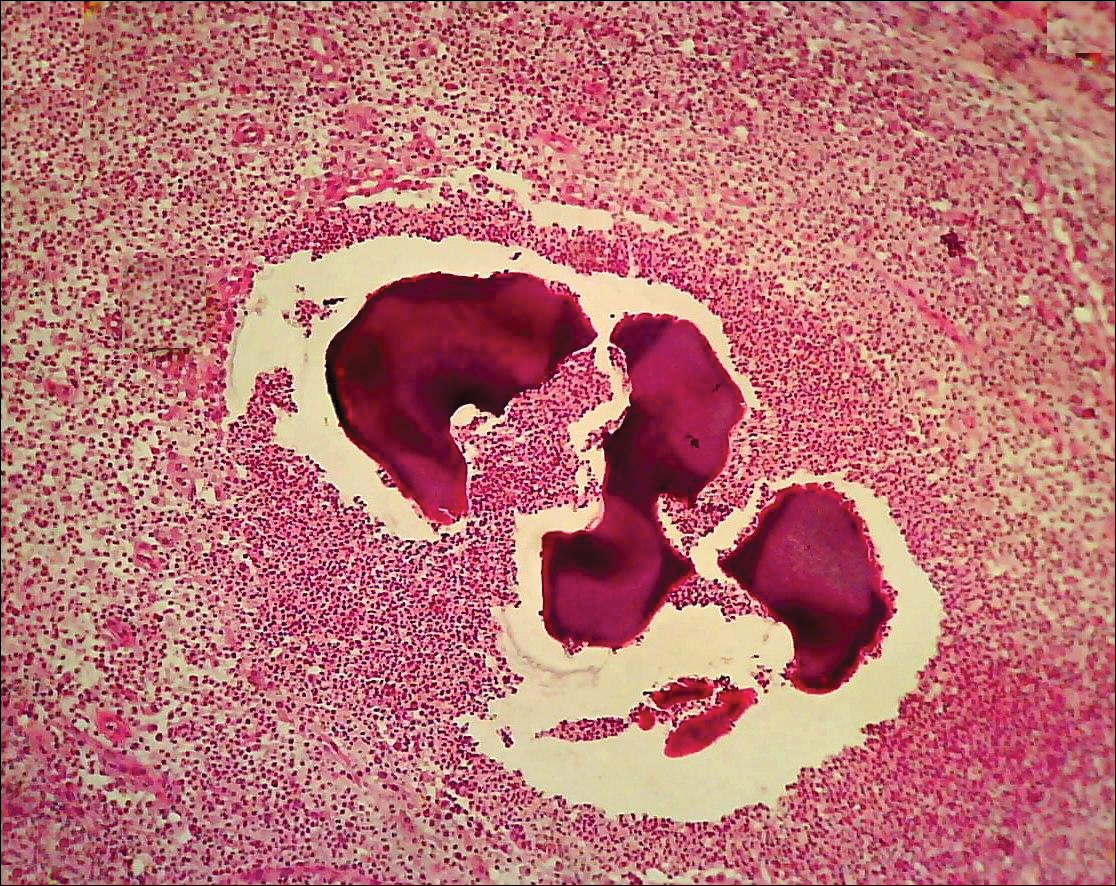
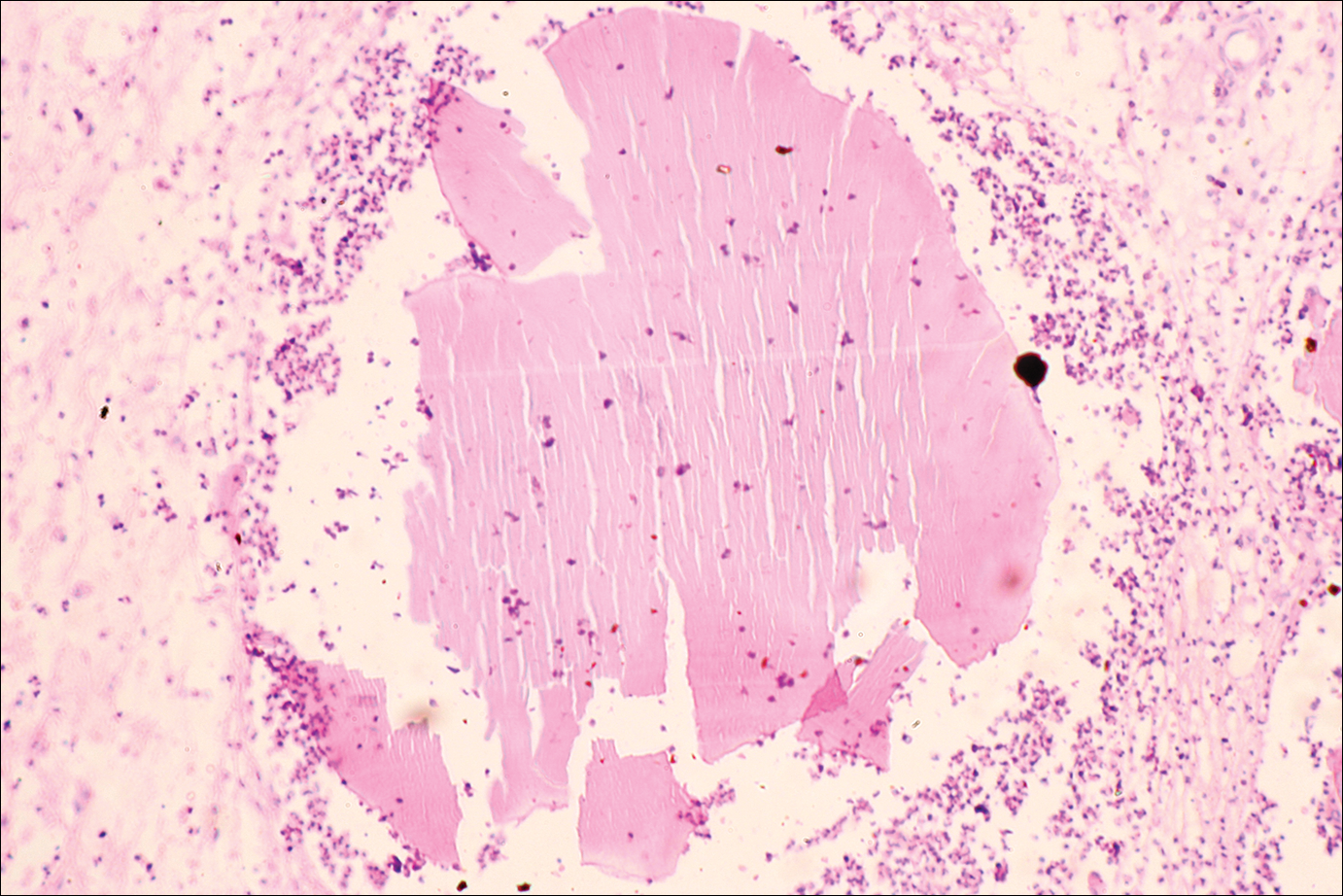
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2

Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
Mycetoma is a subcutaneous disease that can be caused by aerobic bacteria (actinomycetoma) or fungi (eumycetoma). Diagnosis is based on clinical manifestations, including swelling and deformity of affected areas, as well as the presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate that contains the microorganisms.
The worldwide proportion of mycetomas is 60% actinomycetomas and 40% eumycetomas.1 The disease is endemic in tropical, subtropical, and temperate regions, predominating between latitudes 30°N and 15°S. Most cases occur in Africa, especially Sudan, Mauritania, and Senegal; India; Yemen; and Pakistan. In the Americas, the countries with the most reported cases are Mexico and Venezuela.1
Although mycetoma is rare in developed countries, migration of patients from endemic areas makes knowledge of this condition crucial for dermatologists worldwide. We present a review of the current concepts in the epidemiology, clinical presentation, diagnosis, and treatment of actinomycetoma.
Epidemiology
Actinomycetoma is more common in Latin America, with Mexico having the highest incidence. At last count, there were 2631 cases reported in Mexico.2 The majority of cases of mycetoma in Mexico are actinomycetoma (98%), including Nocardia (86%) and Actinomadura madurae (10%). Eumycetoma is rare in Mexico, constituting only 2% of cases.2 Worldwide, men are affected more commonly than women, which is thought to be related to a higher occupational risk during agricultural labor.
Clinical Features
Mycetoma can affect the skin, subcutaneous tissue, bones, and occasionally the internal organs. It is characterized by swelling, deformation of the affected area, and fistulae that drain serosanguineous or purulent exudates.
In Mexico, 60% of cases of mycetoma affect the lower extremities; the feet are the most commonly affected area, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.1 Other sites include the hands, shoulders, and abdominal wall. The head and neck area are seldom affected.3 Mycetoma lesions grow and disseminate locally. Bone lesions are possible depending on the osteophilic affinity of the etiological agent and on the interactions between the fungus and the host’s immune system. In severe advanced cases of mycetoma, the lesions may involve tendons and nerves. Dissemination via blood or lymphatics is extremely rare.4
Diagnosis
Diagnosis of actinomycetoma is suspected based on clinical features and confirmed by direct examination of exudates with Lugol iodine or saline solution. On direct microscopy, actinomycetes are recognized by the production of filaments with a width of 0.5 to 1 μm. On hematoxylin and eosin stain, the small grains of Nocardia appear eosinophilic with a blue center and pink filaments. On Gram stain, actinomycetoma grains show positive branching filaments. Culture of grains recovered from aspirated material or biopsy specimens provides specific etiologic diagnosis. Cultures should be held for at least 4 weeks. Additionally, there are some enzymatic, molecular, and serologic tests available for diagnosis.5-7 Serologic diagnosis is available in a few centers in Mexico and can be helpful in some cases for diagnosis or follow-up during treatment. Antibodies can be determined via enzyme-linked immunosorbent assay, Western blot analysis, immunodiffusion, or counterimmunoelectrophoresis.8
The causative agents of actinomycetoma can be isolated in Sabouraud dextrose agar. Deep wedge biopsies (or puncture aspiration) are useful in observing the diagnostic grains, which can be identified adequately with Gram stain. Grains usually are surrounded and/or infiltrated by neutrophils. The size, form, and color of grains can identify the causative agent.1 The granules of Nocardia are small (80–130 mm) and reniform or wormlike, with club structures in their periphery (Figure 1). Actinomadura madurae is characterized by large, white-yellow granules that can be seen with the naked eye (1–3 mm). On microscopic examination with hematoxylin and eosin stain, these grains are purple and exhibit peripheral pink pseudofilaments (Figure 2).2 The grains of Actinomadura pelletieri are large (1–3 mm) and red or violaceous. They fragment or break easily, giving the appearance of a broken dish (Figure 3). Streptomyces somaliensis forms round grains approximately 0.5 to 1 cm in diameter. These grains stain poorly and are extremely hard. Cutting the grains during processing results in striation, giving them the appearance of a potato chip (Figure 4).2




Treatment of Actinomycetoma
Precise identification of the etiologic agent is essential to provide effective treatment of actinomycetoma. Without treatment, or in resistant cases, progressive osseous and visceral involvement is inevitable.9 Actinomycetoma without osseous involvement usually responds well to medical treatment.
The treatment of choice for actinomycetoma involving Nocardia brasiliensis is a combination of dapsone 100 to 200 mg once daily and trimethoprim-sulfamethoxazole (TMP-SMX) 80/400 to 160/800 mg once daily for 2 to 3 years.10 Other treatments have included the following: (1) amikacin 15 mg/kg or 500 mg intramuscularly twice daily for 3 weeks plus dapsone 100 to 200 mg once daily plus TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years (amikacin, however, is expensive and potentially toxic [nephrotoxicity and ototoxicity] and therefore is used only in resistant cases); (2) dapsone 100 to 200 mg once daily or TMP-SMX 80/400 to 160/800 mg daily for 2 to 3 years plus intramuscular kanamycin 15 mg/kg once daily for 2 weeks at the beginning of treatment, alternating with rest periods to reduce the risk for nephrotoxicity and ototoxicity10; (3) dapsone 1.5 mg/kg orally twice daily plus phosphomycin 500 mg once daily; (4) dapsone 1.5 mg/kg orally twice daily plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month, then the same dose every other day for 1 to 2 months monitoring for ototoxicity; and (5) TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years or rifampicin (15–20 mg/kg/d) plus streptomycin 1 g once daily (14 mg/kg/d) for 1 month at the beginning of treatment, then the same dose every other day for 2 to 3 months until a total dose of 60 g is administered, monitoring for ototoxicity.11 Audiometric tests and creatinine levels must be performed every 5 weeks during the treatment to monitor toxicity.10
The best results for infections with A pelletieri, A madurae, and S somaliensis have been with streptomycin (1 g once daily in adults; 20 mg/kg once daily in children) until a total dose of 50 g is reached in combination with TMP-SMX or dapsone12 (Figure 5). Alternatives for A madurae infections include streptomycin plus oral clofazimine (100 mg once daily), oral rifampicin (300 mg twice daily), oral tetracycline (1 g once daily), oral isoniazid (300–600 mg once daily), or oral minocycline (100 mg twice daily; also effective for A pelletieri).

More recently, other drugs have been used such as carbapenems (eg, imipenem, meropenem), which have wide-spectrum efficacy and are resistant to β-lactamases. Patients should be hospitalized to receive intravenous therapy with imipenem.2 Carbapenems are effective against gram-positive and gram-negative as well as Nocardia species.13,14 Mycetoma that is resistant, severe, or has visceral involvement can be treated with a combination of amikacin and imipenem.15,16 Meropenem is a similar drug that is available as an oral formulation. Both imipenem and meropenem are recommended in cases with bone involvement.17,18 Alternatives for resistant cases include amoxicillin–clavulanic acid 500/125 mg orally 3 times daily for 3 to 6 months or intravenous cefotaxime 1 g every 8 hours plus intramuscular amikacin 500 mg twice daily plus oral levamisole 300 mg once weekly for 4 weeks.19-23
For resistant cases associated with Nocardia species, clindamycin plus quinolones (eg, ciprofloxacin, moxifloxacin, garenoxacin) at a dose of 25 mg/kg once daily for at least 3 months has been suggested in in vivo studies.23
Overall, the cure rate for actinomycetoma treated with any of the prior therapies ranges from 60% to 90%. Treatment must be modified or stopped if there is clinical or laboratory evidence of drug toxicity.13,24 Surgical treatment of actinomycetoma is contraindicated, as it may cause hematogenous dissemination.
Prognosis
Actinomycetomas of a few months’ duration and without bone involvement respond well to therapy. If no therapy is provided or if there is resistance, the functional and cosmetic prognosis is poor, mainly for the feet. There is a risk for spine involvement with mycetoma on the back and posterior head. Thoracic lesions may penetrate into the lungs. The muscular fascia impedes the penetration of abdominal lesions, but the inguinal canals can offer a path for intra-abdominal dissemination.4 Advanced cases lead to a poor general condition of patients, difficulty in using affected extremities, and in extreme cases even death.
The criteria used to guide the discontinuation of initial therapy for any mycetoma include a decrease in the volume of the lesion, closure of fistulae, 3 consecutive negative monthly cultures, imaging studies showing bone regeneration, lack of echoes and cavities on echography, and absence of grains on examination of fine-needle aspirates.11 After the initial treatment protocol is finished, most experts recommend continuing treatment with dapsone 100 to 300 mg once daily for several years to prevent recurrence.12
Prevention
Mycetoma is a disease associated with poverty. It could be prevented by improving living conditions and by regular use of shoes in rural populations.2
Conclusion
Mycetoma is a chronic infection that develops after traumatic inoculation of the skin with either true fungi or aerobic actinomycetes. The resultant infections are known as eumycetoma or actinomycetoma, respectively. The etiologic agents can be found in the so-called grains. Black grains suggest a fungal infection, minute white grains suggest Nocardia, and red grains are due to A pelletieri. Larger white grains or yellow-white grains may be fungal or actinomycotic in origin.
Specific diagnosis requires direct examination, culture, and biopsy. The treatment of choice for actinomycetoma by N brasiliensis is a combination of dapsone 100 to 200 mg once daily and TMP-SMX 80/400 to 160/800 mg once daily for 2 to 3 years. Other effective treatments include aminoglycosides (eg, amikacine, streptomycin) and quinolones. More recently, some other agents have been used such as carbapenems and natural products of Streptomyces cattleya (imipenem), which have wide-spectrum efficacy and are resistant to β-lactamases.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R. Micología Medica Ilustrada. 4th ed. Mexico City, Mexico: McGraw-Hill Interamericana; 2011:125-146.
- McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97-104.
- Fahal AH. Mycetoma: Clinico-pathological Monograph. Khartoum, Sudan: University of Khartoum Press; 2006:20-23, 81-82.
- Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Int J Dermatol. 2009;48:401-403.
- Chávez G, Arenas R, Pérez-Polito A, et al. Eumycetic mycetoma due to Madurella mycetomatis. report of six cases. Rev Iberoam Micol. 1998;15:90-93.
- Vasquez del Mercado E, Arenas R, Moreno G. Sequelae and long-term consequences of systemic and subcutaneous mycoses. In: Fratamico PM, Smith JL, Brogden KA, eds. Sequelae and Long-term Consequences of Infectious Diseases. Washington, DC: ASM Press; 2009:415-420.
- Mancini N, Ossi CM, Perotti M, et al. Molecular mycological diagnosis and correct antimycotic treatments. J Clin Microbiol. 2005;43:3584-3585.
- Arenas R, Lavalle P. Micetoma (madura foot). In: Arenas R, Estrada R, eds. Tropical Dermatology. Austin, TX: Landes Bioscience; 2001:51-61.
- Welsh O, Sauceda E, González J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Fahal AH. Mycetoma: clinico-pathological monograph. In: Fahal AH. Evidence Based Guidelines for the Management of Mycetoma Patients. Khartoum, Sudan: University of Khartoum Press; 2002:5-15.
- Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47-71.
- Valle ACF, Welsh O, Vera-Cabrera L. Subcutaneous mycoses—mycetoma. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:197-200.
- Fuentes A, Arenas R, Reyes M, et al. Actinomicetoma por Nocardia sp. Informe de cinco casos tratados con imipenem solo o combinado con amikacina. Gac Med Mex. 2006;142:247-252.
- Gombert ME, Aulicino TM, DuBouchet L, et al. Therapy of experimental cerebral nocardiosis with imipenem, amikacin, trimethoprim-sulfamethoxazole, and minocylina. Antimicrob Agents Chemother. 1986;30:270-273.
- Calandra GB, Ricci FM, Wang C, et al. Safety and tolerance comparison of imipenem-cilastatin to cephalotin and cefazolin. J Antimicrob Chemother. 1983;12:125-131.
- Ameen M, Arenas R, Vasquez del Mercado E, et al. Efficacy of imipenem therapy for Nocardia actinomycetomas refractory to sulfonamides. J Am Acad Dermatol. 2010;62:239-246.
- Ameen M, Vargas F, Vasquez del Mercado E, et al. Successful treatment of Nocardia actinomycetoma with meropenem and amikacin combination therapy. Int J Dermatol. 2011;50:443-445.
- Ameen M, Arenas R. Emerging therapeutic regimes for the management of mycetomas. Expert Opin Pharmacother. 2008;9:2077-2085.
- Vera-Cabrera L, Daw-Garza A, Said-Fernández S, et al. Therapeutic effect of a novel oxazolidinone, DA-7867 in BALB/c mice infected with Nocardia brasiliensis. PloS Negl Trop Dis. 2008;2:e289.
- Gómez A, Saúl A, Bonifaz A. Amoxicillin and clavulanic acid in the treatment of actinomicetoma. Int J Dermatol. 1993;32:218-220.
- Méndez-Tovar L, Serrano-Jaen L, Almeida-Arvizu VM. Cefotaxima mas amikacina asociadas a inmunomodulación en el tratamiento de actinomicetoma resistente a tratamiento convencional. Gac Med Mex. 1999;135:517-521.
- Chacon-Moreno BE, Welsh O, Cavazos-Rocha N, et al. Efficacy of ciprofloxacin and moxifloxacin against Nocardia brasiliensis in vitro in an experimental model of actinomycetoma in BALB/c mice. Antimicrob Agents Chemother. 2009;53:295-297.
- Welsh O. Treatment of actinomycetoma. Arch Med Res. 1993;24:413-415.
Practice Points
- Diagnosis of actinomycetoma is based on clinical manifestations including increased swelling and deformity of affected areas, presence of granulation tissue, scars, abscesses, sinus tracts, and a purulent exudate containing microorganisms.
- The feet are the most commonly affected location, followed by the trunk (back and chest), arms, forearms, legs, knees, and thighs.
- Specific diagnosis of actinomycetoma requires clinical examination as well as direct examination of culture and biopsy results.
- Overall, the cure rate for actinomycetoma ranges from 60% to 90%.
Cervical Trophic Syndrome: A Distinct Clinical Entity?
To the Editor:
Samarin et al1 reported a case of superficial cervical ulceration that was proposed to be unilateral ulcerations restricted to a dermatome, representing a new clinical variant in the spectrum of trigeminal trophic syndrome (TTS) termed cervical trophic syndrome. The patient was a cognitively impaired elderly woman with a superficial ulceration on the posterior aspect of the neck and a history of herpes zoster (HZ) infection of the affected dermatome.1 We report a similar case of a patient with underlying spinal pathology and discuss these cases as a spectrum between TTS and notalgia paresthetica whereby lesions of peripheral spinal nerves may lead to unilateral ulcerations restricted to a dermatome.
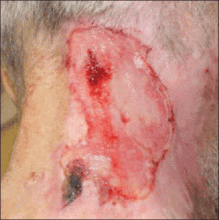
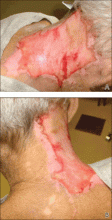
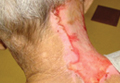
A 66-year-old man with a history of mental retardation and end-stage renal disease presented with an ulceration on the posterior aspect of the neck of approximately 9 months’ duration. Cervical and thoracic radiographs showed levoscoliosis and moderate degenerative disc disease as well as facet arthropathy in the cervical spine. His history was negative for HZ infection. The patient admitted to a chronic inability to control scratching of the area. On examination a 15×15-cm, unilateral, shallow ulceration was seen, with sharp midline demarcation involving the C3-C5 dermatomes (Figure 1). He underwent debridement with general anesthesia, daily dressing changes, and a renal dose of gabapentin 100 mg twice daily. During treatment, 2 biopsies were unremarkable and only showed ulceration and fibrosis. He continued to make progress over the next 2 months with successive in-office debridement and wound care therapy (Figure 2). Unfortunately, he died of an unrelated cause during follow-up.
Our patient’s history and presentation are best explained as a variant of notalgia paresthetica complicated by self-mutilation. Notalgia paresthetica typically presents as unilateral pruritus over the scapular border of the upper back. The classic distribution of pruritus of the T2-T6 dermatomes is strongly associated with degenerative spinal changes; however, degeneration solely confined to cervical segments has been described in etiologic investigations of notalgia paresthetica.2,3 Our case and the case reported by Samarin et al1 involved the C3-C5 dermatomes. Although cases of pruritus confined to the neck also have been previously classified as brachioradial pruritus, the cases described by Wallengren and Sundler4 were proposed to have predisposing spinal disease as well as a component of solar-induced nerve injury. Because cervical ulceration restricted to a dermatome was present in the case described by Samarin et al,1 it appeared to be associated with antecedent HZ infection and was suggested to represent a new clinical syndrome or variant of TTS. Although classically due to mechanical ablation of the trigeminal ganglion, TTS also may be secondary to vascular or viral insult to the trigeminal ganglion or nerve.5,6 The etiology typically is presumed to be secondary to self-mutilation due to underlying paresthesia or dysesthesia. The trigeminal ganglion only is unique in size and location when compared to spinal dorsal root ganglia.
These 2 cases suggest that any insult to any sensory nerve, whether compression related, viral, or vascular, can produce trophic ulcerations secondary to pruritus or self-mutilation. It is likely that TTS is more commonly encountered than other spinal trophic lesions due to the size and location of the trigeminal nerve and ganglion.
Ulceration is not a typical feature of notalgia paresthetica or brachioradial pruritus; a history of self-mutilation due to underlying paresthesia is consistent with the diagnosis. These 2 cases represent notalgia paresthetica and postherpetic pruritus in patients with extreme self-mutilative responses to previously described neuropathic itch syndromes. Spinal trophic syndromes likely are a spectrum of disorders in continuum with TTS.
1. Samarin FM, Ko CJ, Bolognia JL. Cervical trophic syndrome. J Am Acad Dermatol. 2010;63:724-725.
2. Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
3. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:085-1087.
4. Wallengren J, Sundler F. Brachioradial pruritus is associated with a reduction in cutaneous innervation that normalizes during the symptom-free remissions. J Am Acad Dermatol. 2005;52:142-145.
5. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
6. Kavanagh GM, Tidman MJ, McLaren KM, et al. The trigeminal trophic syndrome: an under-recognized complication. Clin Exp Dermatol. 1996;21:299-301.
To the Editor:
Samarin et al1 reported a case of superficial cervical ulceration that was proposed to be unilateral ulcerations restricted to a dermatome, representing a new clinical variant in the spectrum of trigeminal trophic syndrome (TTS) termed cervical trophic syndrome. The patient was a cognitively impaired elderly woman with a superficial ulceration on the posterior aspect of the neck and a history of herpes zoster (HZ) infection of the affected dermatome.1 We report a similar case of a patient with underlying spinal pathology and discuss these cases as a spectrum between TTS and notalgia paresthetica whereby lesions of peripheral spinal nerves may lead to unilateral ulcerations restricted to a dermatome.
A 66-year-old man with a history of mental retardation and end-stage renal disease presented with an ulceration on the posterior aspect of the neck of approximately 9 months’ duration. Cervical and thoracic radiographs showed levoscoliosis and moderate degenerative disc disease as well as facet arthropathy in the cervical spine. His history was negative for HZ infection. The patient admitted to a chronic inability to control scratching of the area. On examination a 15×15-cm, unilateral, shallow ulceration was seen, with sharp midline demarcation involving the C3-C5 dermatomes (Figure 1). He underwent debridement with general anesthesia, daily dressing changes, and a renal dose of gabapentin 100 mg twice daily. During treatment, 2 biopsies were unremarkable and only showed ulceration and fibrosis. He continued to make progress over the next 2 months with successive in-office debridement and wound care therapy (Figure 2). Unfortunately, he died of an unrelated cause during follow-up.
Our patient’s history and presentation are best explained as a variant of notalgia paresthetica complicated by self-mutilation. Notalgia paresthetica typically presents as unilateral pruritus over the scapular border of the upper back. The classic distribution of pruritus of the T2-T6 dermatomes is strongly associated with degenerative spinal changes; however, degeneration solely confined to cervical segments has been described in etiologic investigations of notalgia paresthetica.2,3 Our case and the case reported by Samarin et al1 involved the C3-C5 dermatomes. Although cases of pruritus confined to the neck also have been previously classified as brachioradial pruritus, the cases described by Wallengren and Sundler4 were proposed to have predisposing spinal disease as well as a component of solar-induced nerve injury. Because cervical ulceration restricted to a dermatome was present in the case described by Samarin et al,1 it appeared to be associated with antecedent HZ infection and was suggested to represent a new clinical syndrome or variant of TTS. Although classically due to mechanical ablation of the trigeminal ganglion, TTS also may be secondary to vascular or viral insult to the trigeminal ganglion or nerve.5,6 The etiology typically is presumed to be secondary to self-mutilation due to underlying paresthesia or dysesthesia. The trigeminal ganglion only is unique in size and location when compared to spinal dorsal root ganglia.
These 2 cases suggest that any insult to any sensory nerve, whether compression related, viral, or vascular, can produce trophic ulcerations secondary to pruritus or self-mutilation. It is likely that TTS is more commonly encountered than other spinal trophic lesions due to the size and location of the trigeminal nerve and ganglion.
Ulceration is not a typical feature of notalgia paresthetica or brachioradial pruritus; a history of self-mutilation due to underlying paresthesia is consistent with the diagnosis. These 2 cases represent notalgia paresthetica and postherpetic pruritus in patients with extreme self-mutilative responses to previously described neuropathic itch syndromes. Spinal trophic syndromes likely are a spectrum of disorders in continuum with TTS.
To the Editor:
Samarin et al1 reported a case of superficial cervical ulceration that was proposed to be unilateral ulcerations restricted to a dermatome, representing a new clinical variant in the spectrum of trigeminal trophic syndrome (TTS) termed cervical trophic syndrome. The patient was a cognitively impaired elderly woman with a superficial ulceration on the posterior aspect of the neck and a history of herpes zoster (HZ) infection of the affected dermatome.1 We report a similar case of a patient with underlying spinal pathology and discuss these cases as a spectrum between TTS and notalgia paresthetica whereby lesions of peripheral spinal nerves may lead to unilateral ulcerations restricted to a dermatome.
A 66-year-old man with a history of mental retardation and end-stage renal disease presented with an ulceration on the posterior aspect of the neck of approximately 9 months’ duration. Cervical and thoracic radiographs showed levoscoliosis and moderate degenerative disc disease as well as facet arthropathy in the cervical spine. His history was negative for HZ infection. The patient admitted to a chronic inability to control scratching of the area. On examination a 15×15-cm, unilateral, shallow ulceration was seen, with sharp midline demarcation involving the C3-C5 dermatomes (Figure 1). He underwent debridement with general anesthesia, daily dressing changes, and a renal dose of gabapentin 100 mg twice daily. During treatment, 2 biopsies were unremarkable and only showed ulceration and fibrosis. He continued to make progress over the next 2 months with successive in-office debridement and wound care therapy (Figure 2). Unfortunately, he died of an unrelated cause during follow-up.
Our patient’s history and presentation are best explained as a variant of notalgia paresthetica complicated by self-mutilation. Notalgia paresthetica typically presents as unilateral pruritus over the scapular border of the upper back. The classic distribution of pruritus of the T2-T6 dermatomes is strongly associated with degenerative spinal changes; however, degeneration solely confined to cervical segments has been described in etiologic investigations of notalgia paresthetica.2,3 Our case and the case reported by Samarin et al1 involved the C3-C5 dermatomes. Although cases of pruritus confined to the neck also have been previously classified as brachioradial pruritus, the cases described by Wallengren and Sundler4 were proposed to have predisposing spinal disease as well as a component of solar-induced nerve injury. Because cervical ulceration restricted to a dermatome was present in the case described by Samarin et al,1 it appeared to be associated with antecedent HZ infection and was suggested to represent a new clinical syndrome or variant of TTS. Although classically due to mechanical ablation of the trigeminal ganglion, TTS also may be secondary to vascular or viral insult to the trigeminal ganglion or nerve.5,6 The etiology typically is presumed to be secondary to self-mutilation due to underlying paresthesia or dysesthesia. The trigeminal ganglion only is unique in size and location when compared to spinal dorsal root ganglia.
These 2 cases suggest that any insult to any sensory nerve, whether compression related, viral, or vascular, can produce trophic ulcerations secondary to pruritus or self-mutilation. It is likely that TTS is more commonly encountered than other spinal trophic lesions due to the size and location of the trigeminal nerve and ganglion.
Ulceration is not a typical feature of notalgia paresthetica or brachioradial pruritus; a history of self-mutilation due to underlying paresthesia is consistent with the diagnosis. These 2 cases represent notalgia paresthetica and postherpetic pruritus in patients with extreme self-mutilative responses to previously described neuropathic itch syndromes. Spinal trophic syndromes likely are a spectrum of disorders in continuum with TTS.
1. Samarin FM, Ko CJ, Bolognia JL. Cervical trophic syndrome. J Am Acad Dermatol. 2010;63:724-725.
2. Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
3. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:085-1087.
4. Wallengren J, Sundler F. Brachioradial pruritus is associated with a reduction in cutaneous innervation that normalizes during the symptom-free remissions. J Am Acad Dermatol. 2005;52:142-145.
5. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
6. Kavanagh GM, Tidman MJ, McLaren KM, et al. The trigeminal trophic syndrome: an under-recognized complication. Clin Exp Dermatol. 1996;21:299-301.
1. Samarin FM, Ko CJ, Bolognia JL. Cervical trophic syndrome. J Am Acad Dermatol. 2010;63:724-725.
2. Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
3. Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:085-1087.
4. Wallengren J, Sundler F. Brachioradial pruritus is associated with a reduction in cutaneous innervation that normalizes during the symptom-free remissions. J Am Acad Dermatol. 2005;52:142-145.
5. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
6. Kavanagh GM, Tidman MJ, McLaren KM, et al. The trigeminal trophic syndrome: an under-recognized complication. Clin Exp Dermatol. 1996;21:299-301.