User login
A sore and sensitive tongue
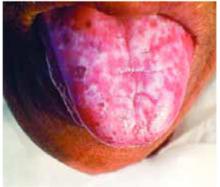
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
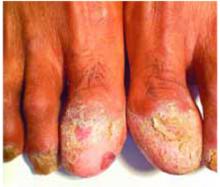
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net.
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net.
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: amorkh@pol.net.
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.
Pigmented lesion on the ear
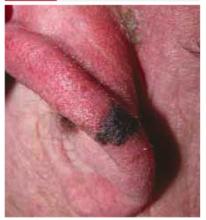
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: amorkh@pol.net.
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: amorkh@pol.net.
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: amorkh@pol.net.
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.