User login
The Power of Two: Revisiting PA Autonomy
PA autonomy is a hot topic lately—not just within the profession but also in the public press. Earlier this year, Forbes touted efforts to loosen “barriers” to PA practice.1 The American Academy of Physician Assistants has identified 10 federal regulations that impose “unnecessary barriers” to medical care provided by PAs; these include cumbersome countersignatures, unnecessary supervision, and ordering restrictions on everything from home care to diabetic shoes.2 Lifting these barriers makes sense amid a shortage of physicians and an influx of newly insured patients (for more on this topic, see here). But as we acquire greater autonomy, we must not lose sight of the relationship that our profession was founded on—and that makes it unique.
Many PAs aspire to be truly independent practitioners, and some already are (eg, in remote and rural areas). Those of us who practice more closely with physicians may be more apt to appreciate the PA-physician partnership. In my 12 years as a PA, I have partnered with more than 50 physicians in cardiology, internal medicine, gastroenterology, and surgical oncology. I have also had the opportunity to witness the dynamic of other PA-physician relationships. As with all relationships, there is a wide range in synergy, productivity, and trust. Some ill-fated relationships are toxic and nonproductive; those merit a swift exit. At the other end of the spectrum are star-crossed partnerships that radiate competence, creativity, and confidence. In between these two extremes are the majority of PA-physician duos, working it out day to day in a kaleidoscopic health care world. It is clear that the physicians and PAs who not only work hard individually, but also work well within their relationship, benefit the most—and so do their patients.
We surgical PAs have a privileged bond that forms at the operating table. It is not only essential, but empowering, to know what each surgeon is thinking about his or her patient as the case unfolds. In medicine, collaboration may not be as intimate as it is in surgery. Still, there is no substitute for knowing our physicians well. Are our treatment goals in sync with theirs? Are we saying the same things to our patients? When we differ (as we often do), how do we achieve respectful and creative dissention? Can we each do our own thing and still be a credible and productive team?
What exactly is PA autonomy? Our scope of practice is delegated by a supervising physician and is limited to the services for which the physician can provide adequate supervision. The terms of supervision vary by state and by practice. In some states, a PA can have multiple “substitute” supervisors, as long as someone keeps a current list. The primary physician supervisor may or may not be the one the PA works with most closely. Some supervisory relationships are merely paper ones, a de rigueur document for the licensing file. Whatever form it takes, supervision has legal and clinical implications that should be implicit to both parties.
If supervision changes drastically, and PA autonomy becomes a reality, the name of our profession would have to change. Physician associate has been proposed. But until we eliminate the word physician, we are still partners with the very profession from which we intend to secede. Certainly, we need to abolish the possessive physician’s (a semantic that rankles universally). Let us get rid of that annoying (and misleading) apostrophe once and for all.
Many of us chose to become PAs to be extenders, rather than bearing ultimate responsibility. That is not to say that we are not willing to make decisions, take risks, or accept liability—that comes with the license and the territory. But in choosing to assist, we have deliberately chosen a dependent role. While physician assistant indeed implies a subordinate relationship, it is not necessarily a subservient one. There is plenty of room for both shared and separate decision-making, for specifically delegated or situational authority. The PA-physician relationship is built on mutual trust that is earned and reinforced on a daily basis. Decisional confidence and technical competence—not wimpy dependence—is the product of a dynamic PA-physician relationship.
As the health care market changes, we can and should afford ourselves every professional opportunity to work as salaried providers or to sign on as single contractors. There are many alternative PA-physician relationships, both legally and financially. If we so desire, we should pursue advanced degrees and specialty certifications and be compensated accordingly. However, multi-tasseled resumes should remain optional. They are costly and not within the reach of everyone, nor do they necessarily make a better PA.
In this new age of autonomy for advanced practice providers, some will say that only a dinosaur could have made these comments. If that is true, thank you for allowing me to wag my tail. The fact remains that PAs offer something unique. In an era of serious physician shortages, we offer patients the opportunity to have both a relationship with their internist, surgeon, or specialist and access to our own capable care and treatment. This is the “package deal” that, sadly, is in danger of becoming overlooked—if not extinct.
1. Japsen B. States remove barriers to physician assistants . Forbes. March 6, 2016. www.forbes.com/sites/brucejapsen/2016/03/06/states-remove-barriers-to-physician-assistants. Accessed September 13, 2016.
2. American Academy of Physician Assistants. Top ten federal laws & regulations imposing unnecessary barriers to medical care provided by PAs. www.aapa.org/workarea/downloadasset.aspx?id=6442450999. Accessed September 13, 2016.
PA autonomy is a hot topic lately—not just within the profession but also in the public press. Earlier this year, Forbes touted efforts to loosen “barriers” to PA practice.1 The American Academy of Physician Assistants has identified 10 federal regulations that impose “unnecessary barriers” to medical care provided by PAs; these include cumbersome countersignatures, unnecessary supervision, and ordering restrictions on everything from home care to diabetic shoes.2 Lifting these barriers makes sense amid a shortage of physicians and an influx of newly insured patients (for more on this topic, see here). But as we acquire greater autonomy, we must not lose sight of the relationship that our profession was founded on—and that makes it unique.
Many PAs aspire to be truly independent practitioners, and some already are (eg, in remote and rural areas). Those of us who practice more closely with physicians may be more apt to appreciate the PA-physician partnership. In my 12 years as a PA, I have partnered with more than 50 physicians in cardiology, internal medicine, gastroenterology, and surgical oncology. I have also had the opportunity to witness the dynamic of other PA-physician relationships. As with all relationships, there is a wide range in synergy, productivity, and trust. Some ill-fated relationships are toxic and nonproductive; those merit a swift exit. At the other end of the spectrum are star-crossed partnerships that radiate competence, creativity, and confidence. In between these two extremes are the majority of PA-physician duos, working it out day to day in a kaleidoscopic health care world. It is clear that the physicians and PAs who not only work hard individually, but also work well within their relationship, benefit the most—and so do their patients.
We surgical PAs have a privileged bond that forms at the operating table. It is not only essential, but empowering, to know what each surgeon is thinking about his or her patient as the case unfolds. In medicine, collaboration may not be as intimate as it is in surgery. Still, there is no substitute for knowing our physicians well. Are our treatment goals in sync with theirs? Are we saying the same things to our patients? When we differ (as we often do), how do we achieve respectful and creative dissention? Can we each do our own thing and still be a credible and productive team?
What exactly is PA autonomy? Our scope of practice is delegated by a supervising physician and is limited to the services for which the physician can provide adequate supervision. The terms of supervision vary by state and by practice. In some states, a PA can have multiple “substitute” supervisors, as long as someone keeps a current list. The primary physician supervisor may or may not be the one the PA works with most closely. Some supervisory relationships are merely paper ones, a de rigueur document for the licensing file. Whatever form it takes, supervision has legal and clinical implications that should be implicit to both parties.
If supervision changes drastically, and PA autonomy becomes a reality, the name of our profession would have to change. Physician associate has been proposed. But until we eliminate the word physician, we are still partners with the very profession from which we intend to secede. Certainly, we need to abolish the possessive physician’s (a semantic that rankles universally). Let us get rid of that annoying (and misleading) apostrophe once and for all.
Many of us chose to become PAs to be extenders, rather than bearing ultimate responsibility. That is not to say that we are not willing to make decisions, take risks, or accept liability—that comes with the license and the territory. But in choosing to assist, we have deliberately chosen a dependent role. While physician assistant indeed implies a subordinate relationship, it is not necessarily a subservient one. There is plenty of room for both shared and separate decision-making, for specifically delegated or situational authority. The PA-physician relationship is built on mutual trust that is earned and reinforced on a daily basis. Decisional confidence and technical competence—not wimpy dependence—is the product of a dynamic PA-physician relationship.
As the health care market changes, we can and should afford ourselves every professional opportunity to work as salaried providers or to sign on as single contractors. There are many alternative PA-physician relationships, both legally and financially. If we so desire, we should pursue advanced degrees and specialty certifications and be compensated accordingly. However, multi-tasseled resumes should remain optional. They are costly and not within the reach of everyone, nor do they necessarily make a better PA.
In this new age of autonomy for advanced practice providers, some will say that only a dinosaur could have made these comments. If that is true, thank you for allowing me to wag my tail. The fact remains that PAs offer something unique. In an era of serious physician shortages, we offer patients the opportunity to have both a relationship with their internist, surgeon, or specialist and access to our own capable care and treatment. This is the “package deal” that, sadly, is in danger of becoming overlooked—if not extinct.
PA autonomy is a hot topic lately—not just within the profession but also in the public press. Earlier this year, Forbes touted efforts to loosen “barriers” to PA practice.1 The American Academy of Physician Assistants has identified 10 federal regulations that impose “unnecessary barriers” to medical care provided by PAs; these include cumbersome countersignatures, unnecessary supervision, and ordering restrictions on everything from home care to diabetic shoes.2 Lifting these barriers makes sense amid a shortage of physicians and an influx of newly insured patients (for more on this topic, see here). But as we acquire greater autonomy, we must not lose sight of the relationship that our profession was founded on—and that makes it unique.
Many PAs aspire to be truly independent practitioners, and some already are (eg, in remote and rural areas). Those of us who practice more closely with physicians may be more apt to appreciate the PA-physician partnership. In my 12 years as a PA, I have partnered with more than 50 physicians in cardiology, internal medicine, gastroenterology, and surgical oncology. I have also had the opportunity to witness the dynamic of other PA-physician relationships. As with all relationships, there is a wide range in synergy, productivity, and trust. Some ill-fated relationships are toxic and nonproductive; those merit a swift exit. At the other end of the spectrum are star-crossed partnerships that radiate competence, creativity, and confidence. In between these two extremes are the majority of PA-physician duos, working it out day to day in a kaleidoscopic health care world. It is clear that the physicians and PAs who not only work hard individually, but also work well within their relationship, benefit the most—and so do their patients.
We surgical PAs have a privileged bond that forms at the operating table. It is not only essential, but empowering, to know what each surgeon is thinking about his or her patient as the case unfolds. In medicine, collaboration may not be as intimate as it is in surgery. Still, there is no substitute for knowing our physicians well. Are our treatment goals in sync with theirs? Are we saying the same things to our patients? When we differ (as we often do), how do we achieve respectful and creative dissention? Can we each do our own thing and still be a credible and productive team?
What exactly is PA autonomy? Our scope of practice is delegated by a supervising physician and is limited to the services for which the physician can provide adequate supervision. The terms of supervision vary by state and by practice. In some states, a PA can have multiple “substitute” supervisors, as long as someone keeps a current list. The primary physician supervisor may or may not be the one the PA works with most closely. Some supervisory relationships are merely paper ones, a de rigueur document for the licensing file. Whatever form it takes, supervision has legal and clinical implications that should be implicit to both parties.
If supervision changes drastically, and PA autonomy becomes a reality, the name of our profession would have to change. Physician associate has been proposed. But until we eliminate the word physician, we are still partners with the very profession from which we intend to secede. Certainly, we need to abolish the possessive physician’s (a semantic that rankles universally). Let us get rid of that annoying (and misleading) apostrophe once and for all.
Many of us chose to become PAs to be extenders, rather than bearing ultimate responsibility. That is not to say that we are not willing to make decisions, take risks, or accept liability—that comes with the license and the territory. But in choosing to assist, we have deliberately chosen a dependent role. While physician assistant indeed implies a subordinate relationship, it is not necessarily a subservient one. There is plenty of room for both shared and separate decision-making, for specifically delegated or situational authority. The PA-physician relationship is built on mutual trust that is earned and reinforced on a daily basis. Decisional confidence and technical competence—not wimpy dependence—is the product of a dynamic PA-physician relationship.
As the health care market changes, we can and should afford ourselves every professional opportunity to work as salaried providers or to sign on as single contractors. There are many alternative PA-physician relationships, both legally and financially. If we so desire, we should pursue advanced degrees and specialty certifications and be compensated accordingly. However, multi-tasseled resumes should remain optional. They are costly and not within the reach of everyone, nor do they necessarily make a better PA.
In this new age of autonomy for advanced practice providers, some will say that only a dinosaur could have made these comments. If that is true, thank you for allowing me to wag my tail. The fact remains that PAs offer something unique. In an era of serious physician shortages, we offer patients the opportunity to have both a relationship with their internist, surgeon, or specialist and access to our own capable care and treatment. This is the “package deal” that, sadly, is in danger of becoming overlooked—if not extinct.
1. Japsen B. States remove barriers to physician assistants . Forbes. March 6, 2016. www.forbes.com/sites/brucejapsen/2016/03/06/states-remove-barriers-to-physician-assistants. Accessed September 13, 2016.
2. American Academy of Physician Assistants. Top ten federal laws & regulations imposing unnecessary barriers to medical care provided by PAs. www.aapa.org/workarea/downloadasset.aspx?id=6442450999. Accessed September 13, 2016.
1. Japsen B. States remove barriers to physician assistants . Forbes. March 6, 2016. www.forbes.com/sites/brucejapsen/2016/03/06/states-remove-barriers-to-physician-assistants. Accessed September 13, 2016.
2. American Academy of Physician Assistants. Top ten federal laws & regulations imposing unnecessary barriers to medical care provided by PAs. www.aapa.org/workarea/downloadasset.aspx?id=6442450999. Accessed September 13, 2016.
Woman, 45, With Red, Scaly Nipple
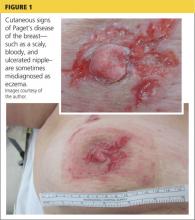
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
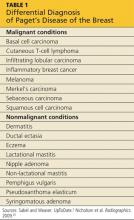
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
Management of Carotid Artery Disease
Carotid artery disease is a treatable cause of ischemic stroke, a potentially devastating event that affects approximately 700,000 Americans each year and results in more than 160,000 deaths.1,2 Stroke-related medical costs, including associated disability, now approach $60 billion per year. Despite advances in treatment, stroke remains the third leading cause of death in the United States.3
As the population ages, stroke prevention has become an increasing challenge for primary care providers. Guiding patients at risk toward the appropriate testing and treatment can offer lifelong benefits. This article will summarize current practice recommendations for screening asymptomatic individuals and for treatment of carotid artery disease using carotid endarterectomy or carotid angioplasty with stenting.
Scope and Screening
Carotid artery stenosis (CAS) is defined as atherosclerotic narrowing of the extracranial carotid arteries. Possibly 20% of ischemic strokes (which represent more than 85% of all strokes) result from CAS, a condition that may or may not be symptomatic.4Symptomatic CAS may be represented by a cerebrovascular accident, a transient ischemic attack, or one of an array of more subtle but enduring neurologic deficits.
The prime risk factor for CAS is prior history of cerebrovascular disease.4 Cardiovascular disease or cigarette smoking doubles a patient’s risk for developing CAS. Other risk factors include age greater than 65, male gender, hypertension, atrial fibrillation, and clotting disorders.
Population studies based on carotid ultrasonography estimate the prevalence of CAS at 0.5% to 8.0% in the general population.4-6 Clinically significant CAS (60% or higher) has been estimated at 1% in those older than 65.4
The degree of carotid occlusion correlates directly with the risk of ipsilateral stroke. The rate of stroke among asymptomatic patients with CAS of at least 80% is 3.5% to 5.0% per year.7 To date, there is no clinically useful risk model to identify those who have CAS or will develop it.
Screening the general population for asymptomatic CAS is not currently recommended.4,8 Guidelines published in 2007 by the Society for Vascular Surgery (SVS)3 advise ultrasound screening only for persons 55 and older who have cardiovascular risk factors, including diabetes, hypertension, hypercholesterolemia, a history of smoking, or known cerebrovascular disease. That same year, the American Society of Neuroimaging9 recommended screening of adults 65 or older who have three or more cardiovascular risk factors.
Ultrasound screening is approximately 94% sensitive and 92% specific for moderate to severe CAS (ie, 60% to 90% occlusion).4 Patients with positive ultrasound findings may next undergo computerized axial angiography, magnetic resonance angiography, or digital subtraction angiography.
Angiography can detect with good precision the degree and location of carotid occlusion, which in turn helps to select treatment options, in consideration of their inherent risks and benefits. These options are medical therapy alone, or medical therapy combined with carotid endarterectomy (CEA), or carotid angioplasty with stenting.
The Research
Stroke prevention, long since a medical priority, is most commonly sought by way of pharmacotherapy combined with lifestyle modification. Surgery, in the form of CEA, also plays an enduring and proven role. Randomized trials, including three landmark studies,10-12 have established CEA as standard treatment for symptomatic and high-grade occlusive carotid disease. The North American Symptomatic Carotid Endarterectomy Trial (NASCET)10 and the European Carotid Surgery Trial (ECST)11 provided the basis for stratifying symptomatic patients and determining whether surgery will produce a reasonable benefit. The Asymptomatic Carotid Atherosclerosis Study (ACAS)12 extended the research to asymptomatic patients with high-grade stenosis.8,13 The benefits of CEA for elderly patients (75 and older) with significant comorbidities were supported in the 2009 New York Carotid Artery Surgery Study (NYCAS).14
Researchers for ACAS,12 which compared medical therapy alone with CEA plus medical therapy in asymptomatic patients with CAS, reported a relative risk (RR) reduction of 0.53 in patients undergoing CEA, with a 5.1% five-year rate of stroke or death in the CEA group versus 11.0% among patients receiving medical therapy alone. The Asymptomatic Carotid Surgery Trial15 (ACST) yielded similar event rates (CEA, 6.4%; medical therapy alone, 11.8%). In both trials, the perioperative (30-day) risk of stroke or death associated with CEA ranged from 2.7% to 3.1%. In the long term (five to 10 years and beyond), RR reduction remains uncertain.
However, CEA remains the gold standard for the treatment of severe carotid artery disease. Currently, about 75% of patients who undergo CEA for significant CAS are asymptomatic.13
Complications associated with CEA occur at an ascending rate, commensurate with the patient’s preoperative stroke history. Researchers for the NYCAS14 reported a 30-day post-CEA rate of stroke or death of nearly 3% among asymptomatic patients with no history of stroke or TIA; nearly 8% among patients with previous stroke; and more than 13% in patients with crescendo TIA or evolving stroke. A significant increase in complications (including stroke or death) was reported among patients with coronary artery disease or with diabetes requiring insulin therapy.
The Case for Carotid Angioplasty with Stenting
Though broadly accepted and practiced, CEA carries significant risk for symptomatic patients and for those who face higher surgical risks, such as diabetes or cardiovascular disease, or anatomic issues such as contralateral occlusions (see Table 1).
Carotid angioplasty with stenting emerged in the 1990s as a less invasive alternative to CEA that could be performed under local anesthesia and with little or no sedation. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),17 no significant difference was found in three-year stroke risk between patients assigned randomly to CEA or to carotid stenting.
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial,7,16,18 an industry-sponsored study, was the first multicenter study to compare CEA with stenting in patients considered at high surgical risk. All stenting procedures were performed using an intraoperative embolic protection device. Patients with stents had a 4.8% risk of stroke, MI, or death in the 30-day postoperative period, compared with 9.8% among patients who underwent CEA. Despite their potential clinical relevance, these results were not found to be statistically significant.
The risk of ipsilateral stroke at one year was similar between treatment groups. Follow-up data published in 2008 and 2009 showed comparable outcomes and no differences in repeat revascularization rates between CEA and stent groups.7,16 SAPPHIRE is now conducting a worldwide registry study in an effort to extend its results to a broader population. The Center for Medicare Services has approved carotid artery stenting with embolic protection for patients who meet the SAPPHIRE high-risk criteria.
Research on the effectiveness of distal protection devices in preventing intraoperative stroke is ongoing.19 In the interim, the SVS recommends embolic protection during all carotid stenting procedures.13 Perioperative medical management remains critical to the success of carotid stenting. This includes intraoperative heparin and clopidogrel for at least two to four weeks postoperatively.7,16
In elderly patients (80 and older), carotid artery stenting may present a particularly high risk.20,21 Investigators for the ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) have found a periprocedural risk of death or stroke at 12.1% among older CAS patients, compared with 4.0% among their younger counterparts.21,22 This discrepancy has been attributed to age-related changes in vasculature that create a more hostile environment for endovascular devices.
Patients younger than 80 with significant but asymptomatic unilateral stenosis who are at average surgical risk are presently the focus of other trials. Now under way, the Carotid Angioplasty and Stenting versus Endarterectomy in Asymptomatic Subjects with Significant Extracranial Carotid Occlusive Disease Trial (ACT I) is the first major trial involving asymptomatic patients, and so far has shown a lower postintervention event rate than reported in smaller previous studies.23 However, these patients are less likely to experience postoperative events than their symptomatic counterparts. Thus, the role of stenting in asymptomatic patients will require long-term follow-up.
To date, CEA retains its gold standard status as the optimum surgical treatment for preventing stroke-associated morbidity and mortality. At the same time, carotid stenting is emerging as an effective and less invasive alternative, especially for patients younger than 80 who are at high perioperative risk for CEA.
Treatment Guidelines
In 2008, the SVS13 issued clinical practice guidelines based on an empirical analysis of the currently available research on carotid stenosis. These guidelines address both medical and anatomic risk and acknowledge the limitations of comparing massive data obtained from robust but independent clinical trials. The SVS authors acknowledge that some terms (eg, high perioperative risk) remain somewhat difficult to define and are subject to practitioners’ interpretation.
In an effort to achieve consensus, the SVS investigators employed the British-based GRADE (Grading of Recommendations, Assessment, Development and Evaluation) system24 to stratify the strength of its recommendations. This system takes into account factors other than the quality of the data, including the reviewers’ values and preferences, and their evaluation of the data as presented (see Table 213).
As the SVS authors note, there is no significant difference to date between outcomes for stenting versus CEA, including death or stroke within 30 days postprocedure and the need for revascularization within three years.13 They conclude, nevertheless, that CEA remains the treatment of choice for asymptomatic patients with moderate to severe stenosis. Symptomatic patients can be stratified based on age and medical and surgical risk when a choice is being made between CEA and stenting. In patients at high risk, lifelong pharmacologic therapy may be safer than either surgical or endovascular treatment.
The Role of Medical Management
Whether or not CEA or stenting is performed, medical therapy plays a crucial role in the management of carotid artery disease. Most patients are placed on aspirin therapy indefinitely unless its use is contraindicated (eg, by risk for gastrointestinal bleeding). The SVS practice guidelines13 incorporate medical therapy, citing joint recommendations issued in 2006 by the American Heart Association and the American Stroke Association (AHA/ASA)1,25 for tight control of hypertension, blood glucose, and elevated cholesterol.
The AHA/ASA researchers also recommend antiplatelet agents (aspirin, clopidogrel, and/or dipyridamole) for patients with a history of TIA or noncardioembolic ischemic stroke. The guidelines advise moderate alcohol consumption, weight reduction for obese patients, and increased physical activity. Smoking cessation remains the sine qua non of vascular disease management.1,25
Conclusion
Primary care providers play a pivotal role in identifying patients at risk for carotid artery disease and educating them about current treatment options. They have an opportunity to take a proactive role in screening patients (age 55 and over) who smoke or who have diabetes, high blood pressure, high cholesterol, or coronary artery disease.
Detection of moderate to severe CAS can lead to timely surgical intervention in asymptomatic individuals who may not realize they are at risk. Attentive medical and lifestyle management enhances the treatment of carotid disease and reduces the risk of stroke, its most devastating consequence.
1. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(6):1583–1633.
2. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. www.americanheart.org/downloadable/heart/1079736729696HDSStats2004UpdateREV3-19-04.pdf. Accessed December 28, 2009.
3. Society for Vascular Surgery. SVS position statement on vascular screenings. www.vascularweb.org/patients/screenings/SVS_Position_Statement_on_Vascular_Screenings.html. Accessed December 28, 2009.
4. Wolff T, Guirguis-Blake J, Miller T, et al. Screening for carotid artery stenosis: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2007;147(12):860- 870.
5. Colgan MP, Strode GR, Sommer JD, et al. Prevalence of asymptomatic carotid disease: results of duplex scanning in 348 unselected volunteers. J Vasc Surg. 1988;8(6):674-678.
6. Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke. 1992;23(6):818-822.
7. Gurm HS, Yadav JS, Fayad P, et al; SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358(15):1572-1579.
8. US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2007;147(12):854-859.
9. Qureshi AI, Alexandrov AV, Tegeler CH, et al. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17(1):19-47.
10. Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30(9):1751-1758.
11. Rothwell PM, Gutnikov SA, Warlow CP; European Carotid Surgery Trial. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34(2):514-23.
12. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421-1428.
13. Hobson RW 2nd, Mackey WC, Ascher E, et al; Society for Vascular Surgery. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48(2):480-486.
14. Halm EA, Tuhrim S, Wang JJ, et al. Risk factors for perioperative death and stroke after carotid endarterectomy: results of the New York Carotid Artery Surgery Study. Stroke. 2009;40(1):221-229.
15. Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST). Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491-1502.
16. Yadav JS, Wholey MH, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators (SAPPHIRE). Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-1501.
17. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357: 1729–1737.
18. Massop D, Dave R, Metzger C, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv. 2009;73(2):129-136.
19. Barbato JE, Dillavou E, Horowitz MB, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47(4):760-765.
20. Suliman A, Greenberg J, Chandra A, et al. Carotid endarterectomy as the criterion standard in high-risk elderly patients. Arch Surg. 2008; 143(8):736-742.
21. Hobson RW 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106-1111.
22. Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50(5):1224-1231.
23. Derdeyn CP. Carotid stenting for asymptomatic carotid stenosis: trial it. Stroke. 2007;38(2 suppl):715-720.
24. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
25. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
Carotid artery disease is a treatable cause of ischemic stroke, a potentially devastating event that affects approximately 700,000 Americans each year and results in more than 160,000 deaths.1,2 Stroke-related medical costs, including associated disability, now approach $60 billion per year. Despite advances in treatment, stroke remains the third leading cause of death in the United States.3
As the population ages, stroke prevention has become an increasing challenge for primary care providers. Guiding patients at risk toward the appropriate testing and treatment can offer lifelong benefits. This article will summarize current practice recommendations for screening asymptomatic individuals and for treatment of carotid artery disease using carotid endarterectomy or carotid angioplasty with stenting.
Scope and Screening
Carotid artery stenosis (CAS) is defined as atherosclerotic narrowing of the extracranial carotid arteries. Possibly 20% of ischemic strokes (which represent more than 85% of all strokes) result from CAS, a condition that may or may not be symptomatic.4Symptomatic CAS may be represented by a cerebrovascular accident, a transient ischemic attack, or one of an array of more subtle but enduring neurologic deficits.
The prime risk factor for CAS is prior history of cerebrovascular disease.4 Cardiovascular disease or cigarette smoking doubles a patient’s risk for developing CAS. Other risk factors include age greater than 65, male gender, hypertension, atrial fibrillation, and clotting disorders.
Population studies based on carotid ultrasonography estimate the prevalence of CAS at 0.5% to 8.0% in the general population.4-6 Clinically significant CAS (60% or higher) has been estimated at 1% in those older than 65.4
The degree of carotid occlusion correlates directly with the risk of ipsilateral stroke. The rate of stroke among asymptomatic patients with CAS of at least 80% is 3.5% to 5.0% per year.7 To date, there is no clinically useful risk model to identify those who have CAS or will develop it.
Screening the general population for asymptomatic CAS is not currently recommended.4,8 Guidelines published in 2007 by the Society for Vascular Surgery (SVS)3 advise ultrasound screening only for persons 55 and older who have cardiovascular risk factors, including diabetes, hypertension, hypercholesterolemia, a history of smoking, or known cerebrovascular disease. That same year, the American Society of Neuroimaging9 recommended screening of adults 65 or older who have three or more cardiovascular risk factors.
Ultrasound screening is approximately 94% sensitive and 92% specific for moderate to severe CAS (ie, 60% to 90% occlusion).4 Patients with positive ultrasound findings may next undergo computerized axial angiography, magnetic resonance angiography, or digital subtraction angiography.
Angiography can detect with good precision the degree and location of carotid occlusion, which in turn helps to select treatment options, in consideration of their inherent risks and benefits. These options are medical therapy alone, or medical therapy combined with carotid endarterectomy (CEA), or carotid angioplasty with stenting.
The Research
Stroke prevention, long since a medical priority, is most commonly sought by way of pharmacotherapy combined with lifestyle modification. Surgery, in the form of CEA, also plays an enduring and proven role. Randomized trials, including three landmark studies,10-12 have established CEA as standard treatment for symptomatic and high-grade occlusive carotid disease. The North American Symptomatic Carotid Endarterectomy Trial (NASCET)10 and the European Carotid Surgery Trial (ECST)11 provided the basis for stratifying symptomatic patients and determining whether surgery will produce a reasonable benefit. The Asymptomatic Carotid Atherosclerosis Study (ACAS)12 extended the research to asymptomatic patients with high-grade stenosis.8,13 The benefits of CEA for elderly patients (75 and older) with significant comorbidities were supported in the 2009 New York Carotid Artery Surgery Study (NYCAS).14
Researchers for ACAS,12 which compared medical therapy alone with CEA plus medical therapy in asymptomatic patients with CAS, reported a relative risk (RR) reduction of 0.53 in patients undergoing CEA, with a 5.1% five-year rate of stroke or death in the CEA group versus 11.0% among patients receiving medical therapy alone. The Asymptomatic Carotid Surgery Trial15 (ACST) yielded similar event rates (CEA, 6.4%; medical therapy alone, 11.8%). In both trials, the perioperative (30-day) risk of stroke or death associated with CEA ranged from 2.7% to 3.1%. In the long term (five to 10 years and beyond), RR reduction remains uncertain.
However, CEA remains the gold standard for the treatment of severe carotid artery disease. Currently, about 75% of patients who undergo CEA for significant CAS are asymptomatic.13
Complications associated with CEA occur at an ascending rate, commensurate with the patient’s preoperative stroke history. Researchers for the NYCAS14 reported a 30-day post-CEA rate of stroke or death of nearly 3% among asymptomatic patients with no history of stroke or TIA; nearly 8% among patients with previous stroke; and more than 13% in patients with crescendo TIA or evolving stroke. A significant increase in complications (including stroke or death) was reported among patients with coronary artery disease or with diabetes requiring insulin therapy.
The Case for Carotid Angioplasty with Stenting
Though broadly accepted and practiced, CEA carries significant risk for symptomatic patients and for those who face higher surgical risks, such as diabetes or cardiovascular disease, or anatomic issues such as contralateral occlusions (see Table 1).
Carotid angioplasty with stenting emerged in the 1990s as a less invasive alternative to CEA that could be performed under local anesthesia and with little or no sedation. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),17 no significant difference was found in three-year stroke risk between patients assigned randomly to CEA or to carotid stenting.
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial,7,16,18 an industry-sponsored study, was the first multicenter study to compare CEA with stenting in patients considered at high surgical risk. All stenting procedures were performed using an intraoperative embolic protection device. Patients with stents had a 4.8% risk of stroke, MI, or death in the 30-day postoperative period, compared with 9.8% among patients who underwent CEA. Despite their potential clinical relevance, these results were not found to be statistically significant.
The risk of ipsilateral stroke at one year was similar between treatment groups. Follow-up data published in 2008 and 2009 showed comparable outcomes and no differences in repeat revascularization rates between CEA and stent groups.7,16 SAPPHIRE is now conducting a worldwide registry study in an effort to extend its results to a broader population. The Center for Medicare Services has approved carotid artery stenting with embolic protection for patients who meet the SAPPHIRE high-risk criteria.
Research on the effectiveness of distal protection devices in preventing intraoperative stroke is ongoing.19 In the interim, the SVS recommends embolic protection during all carotid stenting procedures.13 Perioperative medical management remains critical to the success of carotid stenting. This includes intraoperative heparin and clopidogrel for at least two to four weeks postoperatively.7,16
In elderly patients (80 and older), carotid artery stenting may present a particularly high risk.20,21 Investigators for the ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) have found a periprocedural risk of death or stroke at 12.1% among older CAS patients, compared with 4.0% among their younger counterparts.21,22 This discrepancy has been attributed to age-related changes in vasculature that create a more hostile environment for endovascular devices.
Patients younger than 80 with significant but asymptomatic unilateral stenosis who are at average surgical risk are presently the focus of other trials. Now under way, the Carotid Angioplasty and Stenting versus Endarterectomy in Asymptomatic Subjects with Significant Extracranial Carotid Occlusive Disease Trial (ACT I) is the first major trial involving asymptomatic patients, and so far has shown a lower postintervention event rate than reported in smaller previous studies.23 However, these patients are less likely to experience postoperative events than their symptomatic counterparts. Thus, the role of stenting in asymptomatic patients will require long-term follow-up.
To date, CEA retains its gold standard status as the optimum surgical treatment for preventing stroke-associated morbidity and mortality. At the same time, carotid stenting is emerging as an effective and less invasive alternative, especially for patients younger than 80 who are at high perioperative risk for CEA.
Treatment Guidelines
In 2008, the SVS13 issued clinical practice guidelines based on an empirical analysis of the currently available research on carotid stenosis. These guidelines address both medical and anatomic risk and acknowledge the limitations of comparing massive data obtained from robust but independent clinical trials. The SVS authors acknowledge that some terms (eg, high perioperative risk) remain somewhat difficult to define and are subject to practitioners’ interpretation.
In an effort to achieve consensus, the SVS investigators employed the British-based GRADE (Grading of Recommendations, Assessment, Development and Evaluation) system24 to stratify the strength of its recommendations. This system takes into account factors other than the quality of the data, including the reviewers’ values and preferences, and their evaluation of the data as presented (see Table 213).
As the SVS authors note, there is no significant difference to date between outcomes for stenting versus CEA, including death or stroke within 30 days postprocedure and the need for revascularization within three years.13 They conclude, nevertheless, that CEA remains the treatment of choice for asymptomatic patients with moderate to severe stenosis. Symptomatic patients can be stratified based on age and medical and surgical risk when a choice is being made between CEA and stenting. In patients at high risk, lifelong pharmacologic therapy may be safer than either surgical or endovascular treatment.
The Role of Medical Management
Whether or not CEA or stenting is performed, medical therapy plays a crucial role in the management of carotid artery disease. Most patients are placed on aspirin therapy indefinitely unless its use is contraindicated (eg, by risk for gastrointestinal bleeding). The SVS practice guidelines13 incorporate medical therapy, citing joint recommendations issued in 2006 by the American Heart Association and the American Stroke Association (AHA/ASA)1,25 for tight control of hypertension, blood glucose, and elevated cholesterol.
The AHA/ASA researchers also recommend antiplatelet agents (aspirin, clopidogrel, and/or dipyridamole) for patients with a history of TIA or noncardioembolic ischemic stroke. The guidelines advise moderate alcohol consumption, weight reduction for obese patients, and increased physical activity. Smoking cessation remains the sine qua non of vascular disease management.1,25
Conclusion
Primary care providers play a pivotal role in identifying patients at risk for carotid artery disease and educating them about current treatment options. They have an opportunity to take a proactive role in screening patients (age 55 and over) who smoke or who have diabetes, high blood pressure, high cholesterol, or coronary artery disease.
Detection of moderate to severe CAS can lead to timely surgical intervention in asymptomatic individuals who may not realize they are at risk. Attentive medical and lifestyle management enhances the treatment of carotid disease and reduces the risk of stroke, its most devastating consequence.
Carotid artery disease is a treatable cause of ischemic stroke, a potentially devastating event that affects approximately 700,000 Americans each year and results in more than 160,000 deaths.1,2 Stroke-related medical costs, including associated disability, now approach $60 billion per year. Despite advances in treatment, stroke remains the third leading cause of death in the United States.3
As the population ages, stroke prevention has become an increasing challenge for primary care providers. Guiding patients at risk toward the appropriate testing and treatment can offer lifelong benefits. This article will summarize current practice recommendations for screening asymptomatic individuals and for treatment of carotid artery disease using carotid endarterectomy or carotid angioplasty with stenting.
Scope and Screening
Carotid artery stenosis (CAS) is defined as atherosclerotic narrowing of the extracranial carotid arteries. Possibly 20% of ischemic strokes (which represent more than 85% of all strokes) result from CAS, a condition that may or may not be symptomatic.4Symptomatic CAS may be represented by a cerebrovascular accident, a transient ischemic attack, or one of an array of more subtle but enduring neurologic deficits.
The prime risk factor for CAS is prior history of cerebrovascular disease.4 Cardiovascular disease or cigarette smoking doubles a patient’s risk for developing CAS. Other risk factors include age greater than 65, male gender, hypertension, atrial fibrillation, and clotting disorders.
Population studies based on carotid ultrasonography estimate the prevalence of CAS at 0.5% to 8.0% in the general population.4-6 Clinically significant CAS (60% or higher) has been estimated at 1% in those older than 65.4
The degree of carotid occlusion correlates directly with the risk of ipsilateral stroke. The rate of stroke among asymptomatic patients with CAS of at least 80% is 3.5% to 5.0% per year.7 To date, there is no clinically useful risk model to identify those who have CAS or will develop it.
Screening the general population for asymptomatic CAS is not currently recommended.4,8 Guidelines published in 2007 by the Society for Vascular Surgery (SVS)3 advise ultrasound screening only for persons 55 and older who have cardiovascular risk factors, including diabetes, hypertension, hypercholesterolemia, a history of smoking, or known cerebrovascular disease. That same year, the American Society of Neuroimaging9 recommended screening of adults 65 or older who have three or more cardiovascular risk factors.
Ultrasound screening is approximately 94% sensitive and 92% specific for moderate to severe CAS (ie, 60% to 90% occlusion).4 Patients with positive ultrasound findings may next undergo computerized axial angiography, magnetic resonance angiography, or digital subtraction angiography.
Angiography can detect with good precision the degree and location of carotid occlusion, which in turn helps to select treatment options, in consideration of their inherent risks and benefits. These options are medical therapy alone, or medical therapy combined with carotid endarterectomy (CEA), or carotid angioplasty with stenting.
The Research
Stroke prevention, long since a medical priority, is most commonly sought by way of pharmacotherapy combined with lifestyle modification. Surgery, in the form of CEA, also plays an enduring and proven role. Randomized trials, including three landmark studies,10-12 have established CEA as standard treatment for symptomatic and high-grade occlusive carotid disease. The North American Symptomatic Carotid Endarterectomy Trial (NASCET)10 and the European Carotid Surgery Trial (ECST)11 provided the basis for stratifying symptomatic patients and determining whether surgery will produce a reasonable benefit. The Asymptomatic Carotid Atherosclerosis Study (ACAS)12 extended the research to asymptomatic patients with high-grade stenosis.8,13 The benefits of CEA for elderly patients (75 and older) with significant comorbidities were supported in the 2009 New York Carotid Artery Surgery Study (NYCAS).14
Researchers for ACAS,12 which compared medical therapy alone with CEA plus medical therapy in asymptomatic patients with CAS, reported a relative risk (RR) reduction of 0.53 in patients undergoing CEA, with a 5.1% five-year rate of stroke or death in the CEA group versus 11.0% among patients receiving medical therapy alone. The Asymptomatic Carotid Surgery Trial15 (ACST) yielded similar event rates (CEA, 6.4%; medical therapy alone, 11.8%). In both trials, the perioperative (30-day) risk of stroke or death associated with CEA ranged from 2.7% to 3.1%. In the long term (five to 10 years and beyond), RR reduction remains uncertain.
However, CEA remains the gold standard for the treatment of severe carotid artery disease. Currently, about 75% of patients who undergo CEA for significant CAS are asymptomatic.13
Complications associated with CEA occur at an ascending rate, commensurate with the patient’s preoperative stroke history. Researchers for the NYCAS14 reported a 30-day post-CEA rate of stroke or death of nearly 3% among asymptomatic patients with no history of stroke or TIA; nearly 8% among patients with previous stroke; and more than 13% in patients with crescendo TIA or evolving stroke. A significant increase in complications (including stroke or death) was reported among patients with coronary artery disease or with diabetes requiring insulin therapy.
The Case for Carotid Angioplasty with Stenting
Though broadly accepted and practiced, CEA carries significant risk for symptomatic patients and for those who face higher surgical risks, such as diabetes or cardiovascular disease, or anatomic issues such as contralateral occlusions (see Table 1).
Carotid angioplasty with stenting emerged in the 1990s as a less invasive alternative to CEA that could be performed under local anesthesia and with little or no sedation. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),17 no significant difference was found in three-year stroke risk between patients assigned randomly to CEA or to carotid stenting.
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial,7,16,18 an industry-sponsored study, was the first multicenter study to compare CEA with stenting in patients considered at high surgical risk. All stenting procedures were performed using an intraoperative embolic protection device. Patients with stents had a 4.8% risk of stroke, MI, or death in the 30-day postoperative period, compared with 9.8% among patients who underwent CEA. Despite their potential clinical relevance, these results were not found to be statistically significant.
The risk of ipsilateral stroke at one year was similar between treatment groups. Follow-up data published in 2008 and 2009 showed comparable outcomes and no differences in repeat revascularization rates between CEA and stent groups.7,16 SAPPHIRE is now conducting a worldwide registry study in an effort to extend its results to a broader population. The Center for Medicare Services has approved carotid artery stenting with embolic protection for patients who meet the SAPPHIRE high-risk criteria.
Research on the effectiveness of distal protection devices in preventing intraoperative stroke is ongoing.19 In the interim, the SVS recommends embolic protection during all carotid stenting procedures.13 Perioperative medical management remains critical to the success of carotid stenting. This includes intraoperative heparin and clopidogrel for at least two to four weeks postoperatively.7,16
In elderly patients (80 and older), carotid artery stenting may present a particularly high risk.20,21 Investigators for the ongoing Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) have found a periprocedural risk of death or stroke at 12.1% among older CAS patients, compared with 4.0% among their younger counterparts.21,22 This discrepancy has been attributed to age-related changes in vasculature that create a more hostile environment for endovascular devices.
Patients younger than 80 with significant but asymptomatic unilateral stenosis who are at average surgical risk are presently the focus of other trials. Now under way, the Carotid Angioplasty and Stenting versus Endarterectomy in Asymptomatic Subjects with Significant Extracranial Carotid Occlusive Disease Trial (ACT I) is the first major trial involving asymptomatic patients, and so far has shown a lower postintervention event rate than reported in smaller previous studies.23 However, these patients are less likely to experience postoperative events than their symptomatic counterparts. Thus, the role of stenting in asymptomatic patients will require long-term follow-up.
To date, CEA retains its gold standard status as the optimum surgical treatment for preventing stroke-associated morbidity and mortality. At the same time, carotid stenting is emerging as an effective and less invasive alternative, especially for patients younger than 80 who are at high perioperative risk for CEA.
Treatment Guidelines
In 2008, the SVS13 issued clinical practice guidelines based on an empirical analysis of the currently available research on carotid stenosis. These guidelines address both medical and anatomic risk and acknowledge the limitations of comparing massive data obtained from robust but independent clinical trials. The SVS authors acknowledge that some terms (eg, high perioperative risk) remain somewhat difficult to define and are subject to practitioners’ interpretation.
In an effort to achieve consensus, the SVS investigators employed the British-based GRADE (Grading of Recommendations, Assessment, Development and Evaluation) system24 to stratify the strength of its recommendations. This system takes into account factors other than the quality of the data, including the reviewers’ values and preferences, and their evaluation of the data as presented (see Table 213).
As the SVS authors note, there is no significant difference to date between outcomes for stenting versus CEA, including death or stroke within 30 days postprocedure and the need for revascularization within three years.13 They conclude, nevertheless, that CEA remains the treatment of choice for asymptomatic patients with moderate to severe stenosis. Symptomatic patients can be stratified based on age and medical and surgical risk when a choice is being made between CEA and stenting. In patients at high risk, lifelong pharmacologic therapy may be safer than either surgical or endovascular treatment.
The Role of Medical Management
Whether or not CEA or stenting is performed, medical therapy plays a crucial role in the management of carotid artery disease. Most patients are placed on aspirin therapy indefinitely unless its use is contraindicated (eg, by risk for gastrointestinal bleeding). The SVS practice guidelines13 incorporate medical therapy, citing joint recommendations issued in 2006 by the American Heart Association and the American Stroke Association (AHA/ASA)1,25 for tight control of hypertension, blood glucose, and elevated cholesterol.
The AHA/ASA researchers also recommend antiplatelet agents (aspirin, clopidogrel, and/or dipyridamole) for patients with a history of TIA or noncardioembolic ischemic stroke. The guidelines advise moderate alcohol consumption, weight reduction for obese patients, and increased physical activity. Smoking cessation remains the sine qua non of vascular disease management.1,25
Conclusion
Primary care providers play a pivotal role in identifying patients at risk for carotid artery disease and educating them about current treatment options. They have an opportunity to take a proactive role in screening patients (age 55 and over) who smoke or who have diabetes, high blood pressure, high cholesterol, or coronary artery disease.
Detection of moderate to severe CAS can lead to timely surgical intervention in asymptomatic individuals who may not realize they are at risk. Attentive medical and lifestyle management enhances the treatment of carotid disease and reduces the risk of stroke, its most devastating consequence.
1. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(6):1583–1633.
2. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. www.americanheart.org/downloadable/heart/1079736729696HDSStats2004UpdateREV3-19-04.pdf. Accessed December 28, 2009.
3. Society for Vascular Surgery. SVS position statement on vascular screenings. www.vascularweb.org/patients/screenings/SVS_Position_Statement_on_Vascular_Screenings.html. Accessed December 28, 2009.
4. Wolff T, Guirguis-Blake J, Miller T, et al. Screening for carotid artery stenosis: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2007;147(12):860- 870.
5. Colgan MP, Strode GR, Sommer JD, et al. Prevalence of asymptomatic carotid disease: results of duplex scanning in 348 unselected volunteers. J Vasc Surg. 1988;8(6):674-678.
6. Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke. 1992;23(6):818-822.
7. Gurm HS, Yadav JS, Fayad P, et al; SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358(15):1572-1579.
8. US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2007;147(12):854-859.
9. Qureshi AI, Alexandrov AV, Tegeler CH, et al. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17(1):19-47.
10. Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30(9):1751-1758.
11. Rothwell PM, Gutnikov SA, Warlow CP; European Carotid Surgery Trial. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34(2):514-23.
12. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421-1428.
13. Hobson RW 2nd, Mackey WC, Ascher E, et al; Society for Vascular Surgery. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48(2):480-486.
14. Halm EA, Tuhrim S, Wang JJ, et al. Risk factors for perioperative death and stroke after carotid endarterectomy: results of the New York Carotid Artery Surgery Study. Stroke. 2009;40(1):221-229.
15. Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST). Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491-1502.
16. Yadav JS, Wholey MH, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators (SAPPHIRE). Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-1501.
17. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357: 1729–1737.
18. Massop D, Dave R, Metzger C, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv. 2009;73(2):129-136.
19. Barbato JE, Dillavou E, Horowitz MB, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47(4):760-765.
20. Suliman A, Greenberg J, Chandra A, et al. Carotid endarterectomy as the criterion standard in high-risk elderly patients. Arch Surg. 2008; 143(8):736-742.
21. Hobson RW 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106-1111.
22. Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50(5):1224-1231.
23. Derdeyn CP. Carotid stenting for asymptomatic carotid stenosis: trial it. Stroke. 2007;38(2 suppl):715-720.
24. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
25. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
1. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(6):1583–1633.
2. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. www.americanheart.org/downloadable/heart/1079736729696HDSStats2004UpdateREV3-19-04.pdf. Accessed December 28, 2009.
3. Society for Vascular Surgery. SVS position statement on vascular screenings. www.vascularweb.org/patients/screenings/SVS_Position_Statement_on_Vascular_Screenings.html. Accessed December 28, 2009.
4. Wolff T, Guirguis-Blake J, Miller T, et al. Screening for carotid artery stenosis: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2007;147(12):860- 870.
5. Colgan MP, Strode GR, Sommer JD, et al. Prevalence of asymptomatic carotid disease: results of duplex scanning in 348 unselected volunteers. J Vasc Surg. 1988;8(6):674-678.
6. Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke. 1992;23(6):818-822.
7. Gurm HS, Yadav JS, Fayad P, et al; SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358(15):1572-1579.
8. US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2007;147(12):854-859.
9. Qureshi AI, Alexandrov AV, Tegeler CH, et al. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17(1):19-47.
10. Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30(9):1751-1758.
11. Rothwell PM, Gutnikov SA, Warlow CP; European Carotid Surgery Trial. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34(2):514-23.
12. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421-1428.
13. Hobson RW 2nd, Mackey WC, Ascher E, et al; Society for Vascular Surgery. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48(2):480-486.
14. Halm EA, Tuhrim S, Wang JJ, et al. Risk factors for perioperative death and stroke after carotid endarterectomy: results of the New York Carotid Artery Surgery Study. Stroke. 2009;40(1):221-229.
15. Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST). Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491-1502.
16. Yadav JS, Wholey MH, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators (SAPPHIRE). Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-1501.
17. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357: 1729–1737.
18. Massop D, Dave R, Metzger C, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv. 2009;73(2):129-136.
19. Barbato JE, Dillavou E, Horowitz MB, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg. 2008;47(4):760-765.
20. Suliman A, Greenberg J, Chandra A, et al. Carotid endarterectomy as the criterion standard in high-risk elderly patients. Arch Surg. 2008; 143(8):736-742.
21. Hobson RW 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106-1111.
22. Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50(5):1224-1231.
23. Derdeyn CP. Carotid stenting for asymptomatic carotid stenosis: trial it. Stroke. 2007;38(2 suppl):715-720.
24. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
25. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.