User login
What’s Eating You? Mosquitoes (Culicidae)
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1
There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
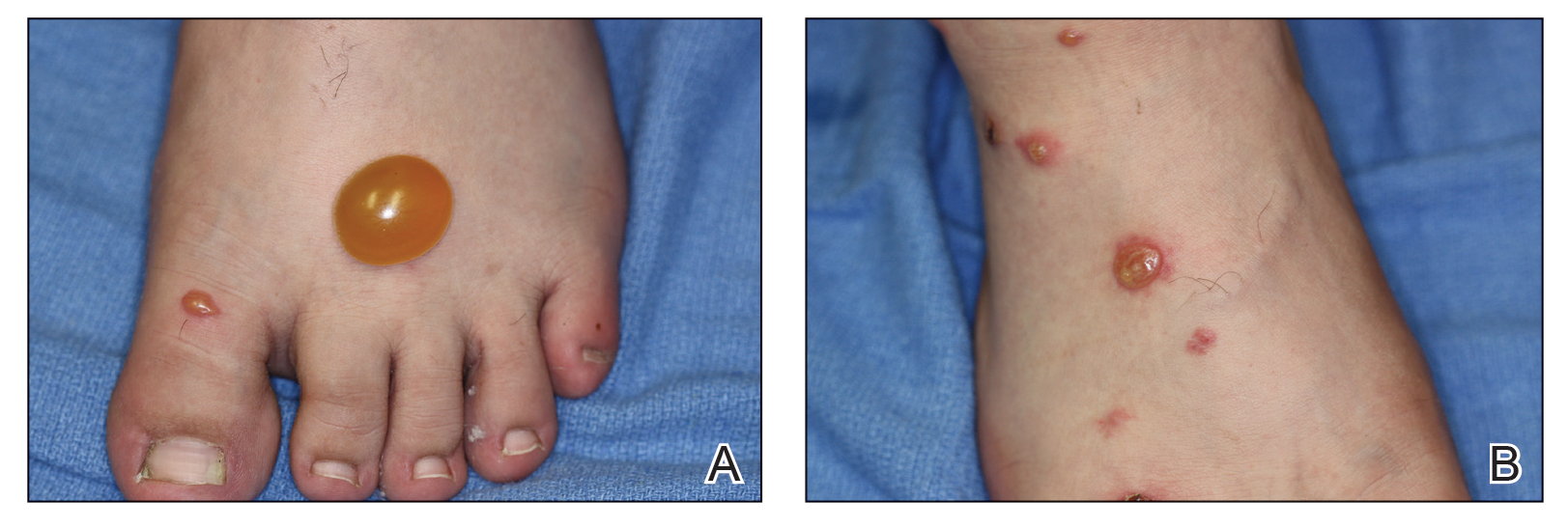
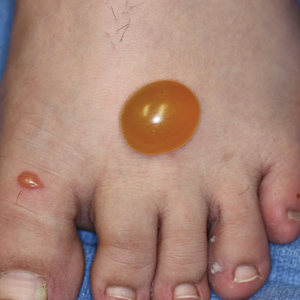
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6
Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1
There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6
Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
Incidence and Characteristics
Mosquitoes are insects categorized into the order of Diptera and family of Culicidae, and more than 3500 different species have been identified.1 In the United States, the most common genus of mosquitoes is Aedes, with other common genera including Culex, Anopheles, Culiseta, and Coquillettidia. Most bites are performed by female rather than male mosquitoes, as it serves to complete their life cycle (Figure 1).1
There are a variety of possible reactions to mosquito bites. Severe local reactions that are large (papules >30 mm in diameter) or are accompanied by systemic manifestations are referred to as hypersensitivity to mosquito bites (HMB).2 These hypersensitivity reactions vary according to multiple factors, including comorbid conditions, genetic predisposition, and geographic location. The majority of the world’s population will exhibit local reactions to mosquito bites at some point during life, with the median age of onset of the first bite at 2 years of age.3 In a study by Arias-Cruz et al,4 the incidence of patient-reported large local reactions was 2.5%. Hypersensitivity to mosquito bites, perhaps the most rare reaction, is more common among Asian and Central American children.5 The median age of diagnosis for HMB is 7 years, and most reactions occur during the first 2 decades of life.6,7
Clinical Presentation
Mosquitoes bite vertebrates in an attempt to feed and thus must locate the host’s blood vessels through a process known as probing, which often necessitates changing the bite site several times. Once the vessel is located and lacerated, the mosquito feeds either from the vessel directly or the hematoma around it. Not only does the bite cause trauma to the skin, but a cutaneous reaction also may occur in response to salivary gland secretions that concurrently are deposited in the host tissue.8 Mosquitoes’ salivary gland components are the primary cause of cutaneous reactions, as one study showed that bites from mosquitoes lacking salivary gland ducts were not associated with these reactions.9 Mosquito saliva contains a large number of compounds with biologic activities, including lysozymes, antibacterial glucosidases, anticoagulants, antiplatelet aggregating factors, and vasodilators, as well as a potentially large number of unknown allergenic proteins. As of 2016, 70 mosquito-derived allergens have been identified, but this number continues to grow.2 After a bite from a mosquito, these compounds may result in host sensitization over time, though interestingly, sensitization to mosquito bites from a species different from the original offender does not occur due to lack of cross-reactivity between species.1
Because mosquitoes reproduce by laying their eggs directly on or near water, people who live near bodies of water or wetlands are at the highest risk for mosquito bites. Patient factors that have been found to lead to increased rates of mosquito bites include lower microbial diversity on the skin, the presence of sweat or body odor, pregnancy, increased body temperature, type O blood, dark clothing, and perfumes.2 Exaggerated bite reactions are associated with Epstein-Barr virus (EBV) infection and hematologic malignancies.10
Immediate hypersensitivity is mediated by a specific IgE antibody and is characterized by erythema and a wheal at the bite site that peaks within minutes of the bite. In contrast, delayed hypersensitivity is lymphocyte mediated; occurs 24 hours after the bite; and causes an indurated, pruritic, and erythematous 2- to 10-mm papule that may blister.11 Although the evidence of immediate hypersensitivity disappears within hours, symptoms of delayed hypersensitivity may last days to weeks. Accompanying symptoms may include local swelling, pain, and warmth. The itch that often is experienced in conjunction with erythema and papule formation is elicited in 3 main ways: direct induction utilizing classic pruritic pathways, IgE-mediated hypersensitivity reaction to salivary components, and IgE-independent host immune response to salivary antigens. Papular urticaria is a common additional finding in children with mosquito bites.1 As an individual is repeatedly bitten, they may undergo 5 stages of sensitization: stage I (neither immediate nor delayed reaction), stage II (delayed reaction), stage III (immediate and delayed reaction), stage IV (immediate reaction), and stage V (neither immediate or delayed reaction).11
Although most mosquito bites cause common local reactions, patients rarely demonstrate systemic reactions that can be much more severe. Skeeter syndrome is a milder systemic response characterized by large local reactions (papules >30 mm in diameter) developing hours after a bite with accompanying fever.12 The reaction typically peaks over days to weeks.2 Although the reaction may resemble cellulitis clinically, a history of a preceding mosquito bite can help make the distinction.13
A more severe systemic reaction is HMB, which is characterized by intense local skin findings as well as generalized systemic symptoms. Initially, indurated, clear, or hemorrhagic bullae appear at the bite site (Figure 2). Later, there is progression to swelling, necrosis, and ulceration.10 Biopsies from the skin lesions associated with HMB reveal necrosis, interstitial and perivascular eosinophilic and lymphocytic infiltrates, and small vessels with fibrinoid necrosis.7 Systemically, high fever, general malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement may occur. Patients typically experience these severe symptoms each time they are bitten.10
The mechanism of the HMB reaction is complex but has a close association with natural killer (NK) cell lymphoproliferative disorder and EBV infection (Figure 3). In fact, it is not uncommon for HMB patients to develop malignant lymphomas during their clinical course, even those unrelated to EBV.14 Epstein-Barr virus, one of the human herpesviruses, produces latent infection in NK cells. It is hypothesized that after a mosquito bite, EBV may be reactivated within these cells by induced expression of the viral lytic-cycle transactivator gene BamHI Z fragment leftward open reading frame 1, BZLF1.6 In response to mosquito salivary gland components, CD4+ T cells proliferate and induce expression of the EBV oncogene latent membrane protein 1, LMP1, on NK cells, which then infiltrate the bite site.15 These EBV-infected NK cells also overexpress the Fas ligand, thus contributing to organ and tissue damage.6 In addition to activating oncogene expression on NK cells, T cells also activate the basophils and mast cells carrying mosquito-specific IgE, both of which also add to the severe skin reaction of HMB.15 The particular triad of HMB, chronic active EBV infection, and NK cell lymphoproliferative disorder commonly is known as HMB-EBV-NK or HEN disease.1 Patients with HMB should be monitored for malignancy. The mortality of HMB is increased in patients in whom onset occurs when they are older than 9 years and with BZLF1 messenger RNA in skin lesions.6
Other rare reactions to mosquito bites include Wells syndrome, anaphylaxis, and superficial lymphangitis. Wells syndrome (also known as eosinophilic cellulitis) is characterized by erythematous or violaceous plaques and pruritic blisters. Although its etiology has not been defined, it is thought to be evoked or exacerbated by insect bites, with CD4+ T cells playing a primary role.1 Anaphylaxis (angioedema, urticaria, and wheezing) rarely may occur due to mosquito salivary gland components but typically is caused by other stinging insects. Superficial lymphangitis, often misdiagnosed as an infection of the lymphatic system, presents within minutes as nontender pink streaks originating from the bite site. A biopsy with eosinophil and mast cell infiltrates consistent with an allergic-type reaction confirms the absence of infection. Patients respond well to glucocorticoid treatment.
Mosquitoes are vectors for many blood-borne diseases, including dengue hemorrhagic fever, malaria, Chikungunya virus, La Crosse encephalitis, St. Louis encephalitis, West Nile virus, and yellow fever.16 Additionally, scratching the bites may lead to superinfection and scarring.1
Prevention and Treatment
Patients with known mosquito sensitivity should avoid areas of stagnant water and utilize preventative measures such as wearing protective clothing and using mosquito repellent containing DEET (N,N-diethyl-meta-toluamide), IR3535 (ethyl butylacetylaminopropionate), picaridin, or 2-undecanone (methyl nonyl ketone or IBI-246) when outdoors. Essential oils such as lemon, eucalyptus, citronella, and garlic are somewhat effective.1 Additionally, prophylactic dosing of antihistamines may prevent milder reactions.
Although often supportive, treatment and management of mosquito bites depends on the extent of the reaction. For common local reactions, symptomatic management with topical anesthetics, calamine lotion, or corticosteroid creams is appropriate. If superinfection from scratching is a concern, antibiotics may be appropriate.
Management of more severe and systemic reactions such as HMB also is supportive, and the addition of oral corticosteroids to decrease inflammation is required.7 Severe HMB also has been treated with immunosuppressive and anticancer drugs, though the efficacy is limited. Venom immunotherapy is a preventative option for patients with mosquito-specific IgE antibodies, and hematopoietic stem cell transplant may be required in patients with HMB.14,16
Conclusion
Mosquito allergens can cause a variety of reactions, ranging from those limited to the skin to those characterized by severe systemic effects. Although common local reactions can be symptomatically treated with topical medication, more severe reactions such as HMB require more involved clinical management. Hypersensitivity to mosquito bites is an important condition to recognize, as it is related to multiple organ impairment as well as later development of malignancy. Patients should be closely monitored during the entire clinical course and in the years following.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
- Fostini AC, Golpanian RS, Rosen JD, et al. Beat the bite: pathophysiology and management of itch in mosquito bites. Itch. 2019;4:1.
- Engler RJ, Crisp HC, Freeman T, et al. Mosquito hypersensitivity: clinical updates. In: Freeman TM, Tracy JM, eds. Stinging Insect Allergy: A Clinician’s Guide. Springer; 2017:203-230.
- Manuyakorn W, Itsaradisaikul S, Benjaponpitak S, et al. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. 2017;35:186-190.
- Arias-Cruz A, Avitia-Valenzuela E, González-Díaz SN, et al. Epidemiology of mosquito bite allergy in the Centre of Allergy and Clinical Immunology of Monterrey, Mexico. J Allergy Clin Immunol. 2006;117:S128.
- Jiang S, Manandhar U, Zheng KP, et al. A case of nodal marginal zone lymphoma with hypersensitivity to mosquito bites as initial symptom. J Cutan Pathol. 2019;46:769-774.
- Kyriakidis I, Vasileiou E, Karastrati S, et al. Primary EBV infection and hypersensitivity to mosquito bites: a case report. Virol Sin. 2016;31:517-520.
- Chiu TM, Lin YM, Wang SC, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. J Microbiol Immunol Infect. 2016;49:613-616.
- Henrique MO, Neto LS, Assis JB, et al. Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes. Immunology. 2019;158:47-59.
- Hudson A, Bowman L, Orr CWM. Effects of absence of saliva on blood feeding by mosquitoes. Science. 1960;131:1730-1731.
- Tatsuno K, Fujiyama T, Matsuoka H, et al. Clinical categories of exaggerated skin reactions to mosquito bites and their pathophysiology. J Dermatol Sci. 2016;82:145-152.
- Oka K, Ohtaki N, Igawa K, et al. Study on the correlation between age and changes in mosquito bite response. J Dermatol. 2018;45:1471-1474.
- Ferdman RM. Superficial allergic lymphangitis with a cutaneous recall reaction to a mosquito bite. Ann Allergy Asthma Immunol. 2019;123:521-522.
- Crisp HS, Johnson KS. Mosquito allergy. Ann Allergy Asthma Immunol. 2013;110:65-69.
- Washio K, Oka T, Abdalkader L, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma. 2017;58:2683-2694.
- Sakakibara Y, Wada T, Muraoka M, et al. Basophil activation by mosquito extracts in patients with hypersensitivity to mosquito bites. Cancer Sci. 2015;106:965-971.
- Lee H, Halvorsen S, Mackey R, et al. Insect allergy. Prim Care. 2016;43:417-431.
Practice Points
- Common local reactions to mosquito bites include immediate and delayed hypersensitivity reactions. With repeated exposure, reactions can increase in severity.
- Hypersensitivity to mosquito bites is a severe systemic reaction to mosquito salivary gland components characterized by bullous necrotic skin lesions associated with systemic manifestations such as high fever, malaise, liver dysfunction, proteinuria, hematuria, hepatosplenomegaly, and lymph node enlargement.
- Hypersensitivity to mosquito bites is closely associated with chronic Epstein-Barr virus infection and lymphoproliferative disorders.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
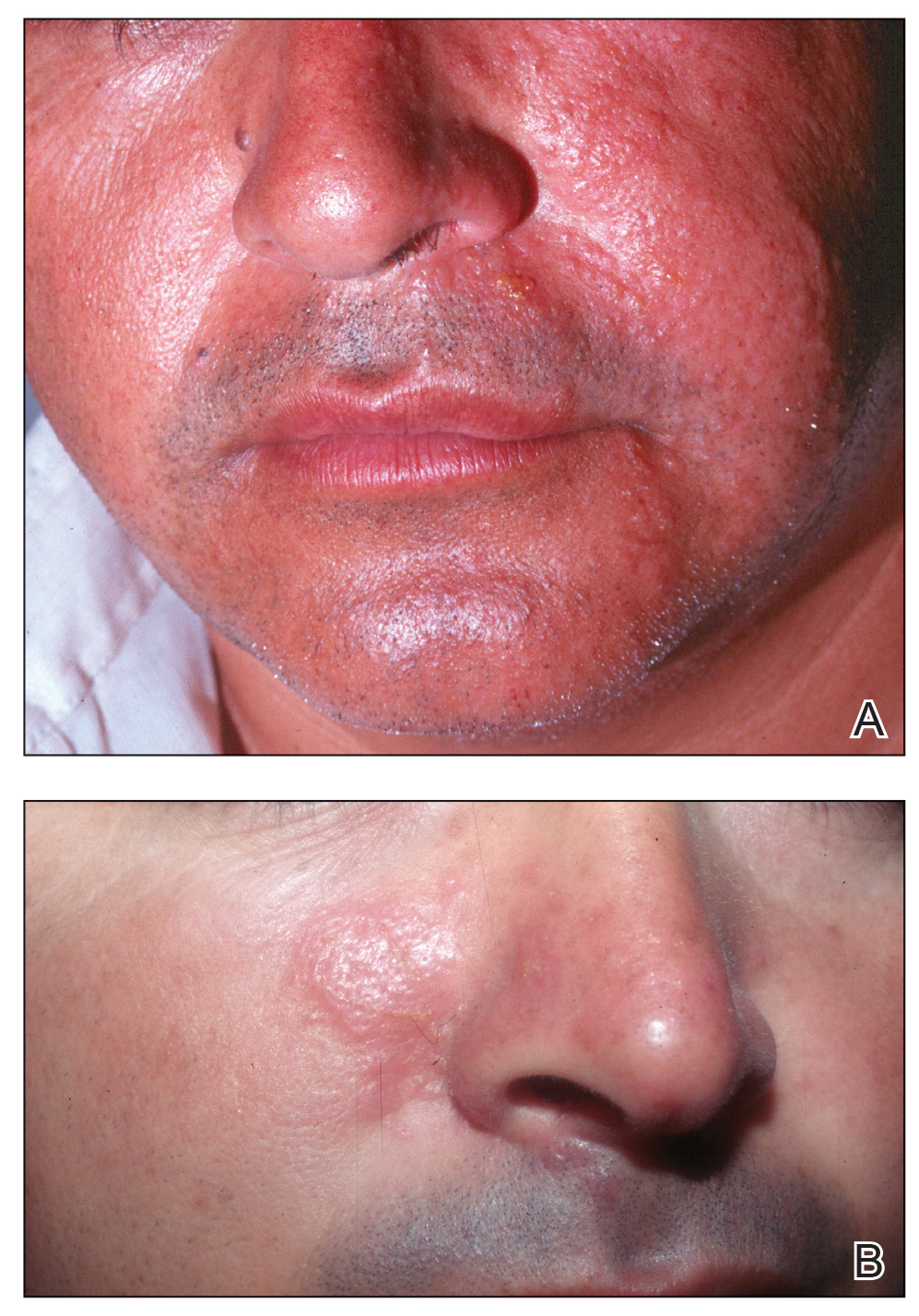
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.