User login
Rule out pulmonary tuberculosis: Clinical and radiographic clues for the internist
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
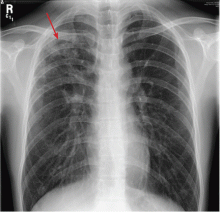
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
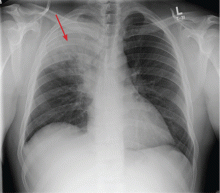
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
Tuberculosis rates in the United States are at an all-time low, which is good news for public health. However, as clinicians see fewer cases of tuberculosis, their skill at making this diagnosis rapidly diminishes.
In 2012, for the first time, fewer than 10,000 tuberculosis cases were reported in the United States to the Centers for Disease Control and Prevention (CDC),1 for a case rate of 3.2 per 100,000. This is in sharp contrast to the worldwide burden of tuberculosis: the World Health Organization2 estimated that there were 8.6 million new cases of tuberculosis in 2012. As a result of travel and immigration, clinicians in the United States will continue to see sporadic cases of active tuberculosis in their hospitals and clinics.
This review describes the clinical and radiographic clues to the diagnosis of pulmonary tuberculosis, discusses the use and discontinuation of respiratory isolation, and reviews the use of new diagnostic technologies.
CASE 1: A COLLEGE STUDENT WITH FATIGUE
A 23-year-old graduate student presents to the student health clinic with vague symptoms of fatigue and several pounds of weight loss over the past 3 months. When asked about coughing, he says he thinks he has had a mild, nonproductive cough for about a month. On examination he is thin, appears comfortable, and has faint rales in the right middle lung zone.
The clinician thinks that the symptoms are likely related to stress, lack of sleep, and difficulty adapting to graduate school life. However, in view of the pulmonary finding on examination, the physician obtains a complete blood cell count (CBC) and a chest radiograph. The CBC is normal. The radiograph (Figure 1) reveals a patchy, somewhat nodular infiltrate in the right upper lobe. The radiologist reviews the results, noting that tuberculosis is high on the list of possible diagnoses. The clinician calls the student and obtains the following additional history.
The patient was born in Thailand and arrived in the United States 3 months ago. Soon after his arrival, he had a tuberculin skin test with purified protein derivative in the student health department, which produced an induration 18 mm in diameter. The patient dismissed this finding as a false-positive result, attributing it to having received BCG vaccine in his native country, and he therefore did not follow up as recommended for a chest radiograph. He denies having fever, night sweats, or hemoptysis.
Since the patient lives in a college dormitory and has four roommates, the clinician admits him to the hospital for further evaluation and for airborne infection isolation. Sputum smears are positive for acid-fast bacilli, and samples ultimately grow Mycobacterium tuberculosis. He is started on standard antituberculosis treatment with isoniazid, rifampin, ethambutol, and pyrazinamide and discharged about 1 week later. He does well. Approximately 50 of his classmates are tested for possible exposure to tuberculosis.
CASE 2: A MAN WITH ACUTE-ONSET SYMPTOMS
A 35-year-old man presents to the emergency department for evaluation of cough with sputum production, fever, nausea, vomiting, and diarrhea. The symptoms began suddenly 1 week previously. He has no medical history, was born in the United States, and works in computer sales. On examination he looks uncomfortable, is slightly tachypneic, and has a temperature of 101°F (38.3°C).
Given his complaint of cough, chest radiography and a CBC are ordered. The white blood cell count is 18.0 × 109/L (reference range 4.5–11.0), with 50% bands (reference range 3%–5%). The chest radiograph (Figure 2) shows a dense infiltrate in the right upper lobe, with air bronchograms and possible right hilar fullness.
The patient is diagnosed with community-acquired pneumonia, and because his oral intake is poor, he is admitted to the hospital and started on azithromycin and ceftriaxone. Blood cultures the next day grow Streptococcus pneumoniae. He fully recovers. A follow-up radiograph is performed 6 weeks later because of the right hilar fullness, and it is normal.
COMMENT
These two cases demonstrate the importance of clinical, demographic, laboratory, and radiographic clues to raise or lower our suspicion for pulmonary tuberculosis. Both patients had right-upper-lobe infiltrates on radiography, yet the diagnosis of tuberculosis was considered only in the first patient.
CLINICAL CLUES TO PULMONARY TUBERCULOSIS
Symptoms of tuberculosis are generally indolent in onset, often so much so that the patient does not realize that he or she is sick until after starting treatment and beginning to improve. In addition, the symptoms can be vague, including only mild fatigue and cough. The classic symptoms of prolonged nonproductive cough, hemoptysis, weight loss, and fever often do not appear until the disease is quite advanced in the lung and the patient has been sick for months.
Since the symptoms of tuberculosis can be nonspecific, the patient’s social and demographic characteristics are important in assessing the likelihood that his or her current illness is tuberculosis.
Foreign birth
Almost two-thirds of all reported tuberculosis cases in the United States are in people who were born outside of the United States.1 The highest risk of reactivation appears to be within the first 5 years after immigration to the United States.3
Other risk factors
- Extensive travel to tuberculosis-endemic regions of the world
- Previous incarceration
- Intravenous drug use
- Work in health care
- Homelessness
- Known exposure to tuberculosis in the past.
Certain medical conditions predispose to reactivation of tuberculosis and should be considered when evaluating someone for active tuberculosis. These include human immunodeficiency virus infection and immunosuppression from tumor necrosis factor inhibitors, steroids, and medications used in organ transplantation. Other risk factors include diabetes requiring insulin, end-stage renal disease, and hematologic malignancies.4 Absence of these risk factors does not exclude tuberculosis, but it decreases the likelihood.
Findings on physical examination and laboratory testing are generally nonspecific in active tuberculosis. In particular, fever is present in 40% to 80% of patients. The white blood cell count is generally normal or only slightly elevated.
Radiographic signs
While the presenting symptoms and physical findings can be nonspecific, there are definite clues to the diagnosis of tuberculosis on chest radiography. In adults, most cases of tuberculosis are reactivation-type, which means the patient was exposed to tuberculosis many months to years in the past.
Reactivation-type tuberculosis usually occurs in the upper lobes, classically in the apical and posterior segments. The infiltrates tend to be patchy rather than densely consolidated. Cavitation, when present, increases the likelihood of tuberculosis. Intrathoracic lymphadenopathy, which occurs in primary tuberculosis, is generally not seen in adults with typical reactivation pulmonary tuberculosis.
However, adults who are highly immunosuppressed, such as those with advanced human immunodeficiency virus infection, organ transplant recipients, or those taking tumor necrosis factor inhibitors, may have radiographic features that are atypical for tuberculosis. For example, they may present with hilar adenopathy or lower-lobe infiltrates.5
Are there clinical prediction rules for tuberculosis?
Because tuberculosis rates have been declining and most hospitals have a limited number of rooms for airborne infection isolation, several studies have evaluated clinical prediction rules for diagnosing pulmonary tuberculosis.
In general, the signs and symptoms that predict tuberculosis are similar to those discussed above, including chronic symptoms, immunosuppression, birth in a region with a high incidence of the disease, a chest radiograph showing upper-zone findings, a positive tuberculin skin test, and fever.6–8 The studies that identified these factors are limited in generalizability as they were performed and validated in single institutions, and the prediction rules have not been widely adopted. Yet they provide a straightforward way to determine which patients should be prioritized for isolation.
RETURN TO THE CASES
The student in case 1 had several features suggesting tuberculosis: indolent and nonspecific symptoms, normal CBC, patchy upper-lobe infiltrates, birth in a country that has a high incidence of tuberculosis, and a positive skin test.
In contrast, the man in case 2 had features that made tuberculosis much less likely: acute symptoms, markedly elevated white blood cell count, and densely consolidated infiltrate. He was also born in the United States and had no additional risk factors for tuberculosis exposure.
EVALUATION OF SUSPECTED TUBERCULOSIS
Who should be admitted to the hospital for evaluation?
In general, a patient with suspected tuberculosis can be evaluated as an outpatient. However, there are a number of reasons to consider hospital admission for the initial workup and for starting treatment:
- Clinical instability requiring inpatient care: eg, hypoxia, unstable vital signs, inability to tolerate oral intake
- Residence in a congregate setting such as a homeless shelter, nursing home, or college dormitory, where there is an ongoing risk of transmitting the infection to others
- Concern that the patient might be lost to follow-up if discharged from the emergency department or clinic
- Vulnerable contacts living in the home with the patient (eg, newborn infants, severely immunosuppressed people)
- Lack of resources in the community to provide prompt evaluation and initiation of treatment; most urban areas have tuberculosis clinics with outreach staff available to provide support for patients, but these resources are scarcer in rural regions.
When should a patient be placed in airborne infection isolation?
Patients with suspected active pulmonary tuberculosis should be placed in airborne infection isolation (also called respiratory or negative-pressure isolation). The purpose of this isolation method is to prevent transmission to other patients and to health care workers. Isolation and other environmental and personal controls such as ultraviolet light and N-95 masks are highly effective in preventing transmission.9
However, there are disadvantages to placing patients in isolation. Only 1 out of every 10 to 25 patients isolated actually has tuberculosis,10 and patients typically remain in isolation for 4 to 7 days. Therefore, unnecessary isolation can delay diagnostic testing for other illnesses and may waste already-limited health care resources. In addition, isolation carries the potential for decreased contact with providers.
When can a patient be released from isolation?
One of the problems with airborne infection isolation is determining when it is safe to discontinue it, especially when the diagnosis of tuberculosis appears less likely.
Traditionally, we have used the requirement of three negative sputum smears for acid-fast bacilli on 3 separate days, as well as low clinical suspicion for tuberculosis. The use of three sputum smears for acid-fast bacilli is based on studies11,12 in populations that have a high prevalence of tuberculosis. These studies found that after three sputum smears were obtained, additional sputum smears were unlikely to improve the sensitivity of the test. The studies focused on maximizing the sensitivity of the test and detecting all potential cases.11,12 However, in US hospitals today, the focus is on rapidly excluding the diagnosis of tuberculosis to minimize hospital length of stay and to allow evaluation for alternative diagnoses.
Several studies have called into question the need for three negative sputum smears to discontinue isolation.13–16 Mathew et al13 found a negative predictive value of 97.8% with a single negative sputum smear for the diagnosis of culture-positive tuberculosis. Each additional sputum increased the negative predictive value by only 0.2%. The authors suggested that one or two negative sputum smears are sufficient to discontinue isolation in a region that has a low incidence of tuberculosis. These studies were all performed at single institutions in the United States and Canada, and their findings are relevant to regions that have a low incidence of tuberculosis.
The CDC continues to recommend that airborne infection isolation be discontinued only when either another diagnosis is made that explains the clinical syndrome or the patient has three negative acid-fast bacilli sputum smear results or two negative acid-fast bacilli smears and one negative nucleic amplification test (discussed below). These should be done at least 8 hours apart and should include at least one early-morning specimen.9
In a minority of cases, empiric treatment for tuberculosis is indicated despite negative sputum smears, based on clinical and radiographic manifestations. Patients receiving empiric treatment for pulmonary tuberculosis should remain in airborne infection isolation during the initiation of treatment (if a hospital stay is required) until cleared by a specialist in infectious disease or tuberculosis.
Can molecular techniques help in rapidly diagnosing tuberculosis?
Additional tests for tuberculosis that are performed on clinical specimens have been available for the past 10 years.
Nucleic acid amplification can detect tuberculosis directly in sputum, bronchoscopy specimens, or other clinical specimens. It is available at reference laboratories, large hospitals, and many state laboratories, often with 24-hour turnaround. Both commercial and in-house tests are performed. The CDC considers nucleic acid amplification to be very helpful and underutilized. An important limitation of the test is that it performs best in smear-positive specimens, with a sensitivity of 96.8%, whereas its sensitivity in smear-negative samples is only 73%.17 For this reason, nucleic acid amplification is still not widely used in US hospitals.
The CDC recommends nucleic acid amplification testing in all patients in whom the diagnosis of tuberculosis is being considered but is not yet confirmed.18 Table 1 outlines the use of nucleic acid amplification in several clinical situations. Use and interpretation of this test in suspected tuberculosis often requires consultation with clinicians who are experienced in the diagnosis of this disease.
How should an interferon-gamma-release assay or a tuberculin skin test be used in evaluating suspected tuberculosis?
Patients who have never tested positive for tuberculosis on a skin test should be tested by tuberculin skin testing or with an interferon-gamma-release assay during an evaluation for suspected pulmonary tuberculosis. The interferon-gamma-release assays available in the United States are the QuantiFERON-TB Gold In-Tube test and the T-Spot TB test. Most larger hospitals have one of the two available.
Of note: up to 25% of patients with active tuberculosis can have a negative skin test or interferon-gamma-release assay at the time of initial diagnosis, the number being higher in those who are immunosuppressed.
An interferon-gamma-release assay, which is performed on the patient’s serum, is preferred in those who have previously received the BCG vaccine, as there is no cross-reactivity between the vaccine and the antigens in the assay. In patients with active tuberculosis, the interferon-gamma-release assay does not perform any better than the skin test, so the choice of test should be determined by availability. Table 2 compares the characteristics of tuberculin skin testing and the interferon- gamma-release assay.19
In evaluating for active tuberculosis, a positive skin test or interferon-gamma-release assay can be helpful in increasing the likelihood of tuberculosis, but a negative result does not exclude active tuberculosis.
Is computed tomography necessary in patients suspected of having active pulmonary tuberculosis?
Additional imaging is often performed in patients with suspected pulmonary tuberculosis, or before the diagnosis of tuberculosis is considered. Computed tomography provides more detailed images of pulmonary infiltrates and may reveal more extensive disease than plain radiography, but the images are not diagnostic. Ultimately, sputum and sometimes tissue are required. Far too often, a sputum smear for acid-fast bacilli is the last test to be performed, after both computed tomography and bronchoscopy have been done. In addition, in order to undergo computed tomography, the patient must be removed from airborne infection isolation.
The decision to perform computed tomography must be individualized to the patient and to the clinical situation. It is certainly not a necessary test for the diagnosis of pulmonary tuberculosis.
When should the diagnosis be reported?
Tuberculosis is a reportable illness in the United States. Although each state varies in its specific requirements, if tuberculosis treatment is being initiated or tuberculosis is strongly suspected, a report should be made to the local public health authority for tuberculosis within 24 hours.
This report allows for outreach services to be offered to the patient, often including directly observed therapy in which doses of antituberculosis treatment are provided and observed to ensure completion of treatment. In addition, public health authorities bear the responsibility for contact investigation to determine if transmission of tuberculosis has occurred in the community.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
- Centers for Disease Control and Prevention (CDC). Trends in tuberculosis—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:201–205.
- World Health Organization (WHO). Tuberculosis. WHO Global Tuberculosis Report 2013. www.who.int/tb/publications/factsheet_global.pdf. Accessed November 13, 2014.
- McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med 1995; 332:1071–1076.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161:S221–S247.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985; 131:393–396.
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict Mycobacterium tuberculosis in inpatients. Arch Intern Med 2000; 160:2471–2476.
- Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 2005; 165:453–457.
- Rakoczy KS, Cohen SH, Nguyen HH. Derivation and validation of a clinical prediction score for isolation of inpatients with suspected pulmonary tuberculosis. Infect Control Hosp Epidemiol 2008; 29:927–932.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54(RR-17):1–141.
- Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 2008; 178:300–305.
- MacGregor RR. A year’s experience with tuberculosis in a private urban teaching hospital in the postsanatorium era. Am J Med 1975; 58:221–228.
- Greenbaum M, Beyt BE Jr, Murray PR. The accuracy of diagnosing pulmonary tuberculosis at a teaching hospital. Am Rev Respir Dis 1980; 121:477–481.
- Mathew P, Kuo YH, Vazirani B, Eng RH, Weinstein MP. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol 2002; 40:3482–3484.
- Bryan CS, Rapp DJ, Brown CA. Discontinuation of respiratory isolation for possible tuberculosis: do two negative sputum smear results suffice? Infect Control Hosp Epidemiol 2006; 27:515–516.
- Nelson SM, Deike MA, Cartwright CP. Value of examining multiple sputum specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol 1998; 36:467–469.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol 2011; 22:e1–e3.
- Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196.
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009; 58:7–10.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59:1–25.
KEY POINTS
- Tuberculosis continues to be in the differential diagnosis for patients hospitalized in the United States.
- Clinical, demographic, and radiologic data obtained during the patient’s initial evaluation are helpful in determining the likelihood of tuberculosis.
- Sputum smears for acid-fast bacilli and either skin testing with purified protein derivative or blood testing with an interferon-gamma-release assay continue to be the mainstays of the initial evaluation for pulmonary tuberculosis.
- Nucleic acid amplification testing of sputum or bronchoscopy specimens can provide additional information and should be considered when pulmonary tuberculosis is part of the differential diagnosis.