User login
Fever, abdominal pain, and adnexal mass
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
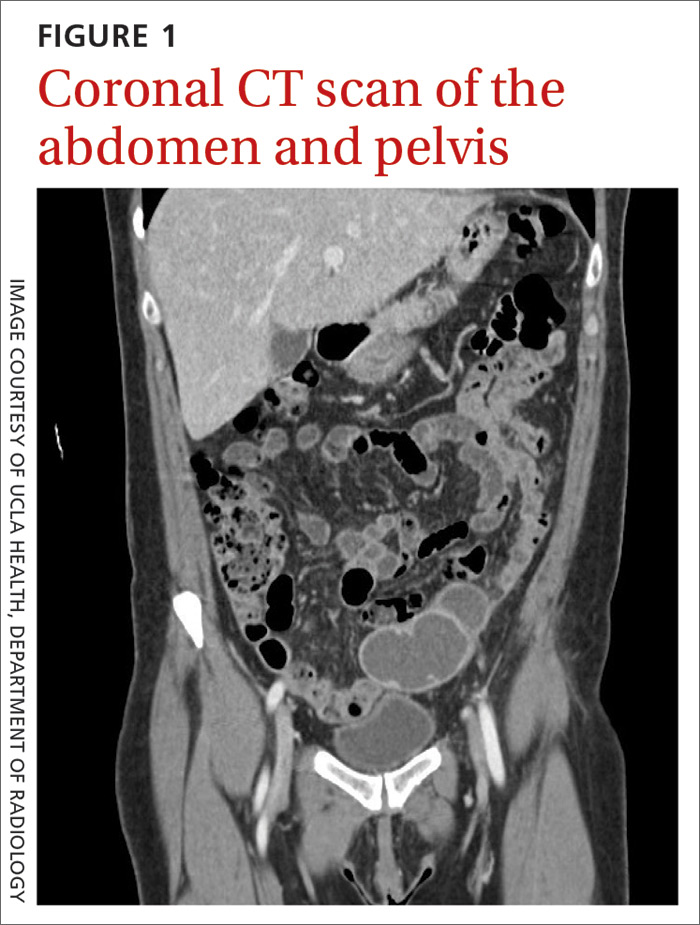
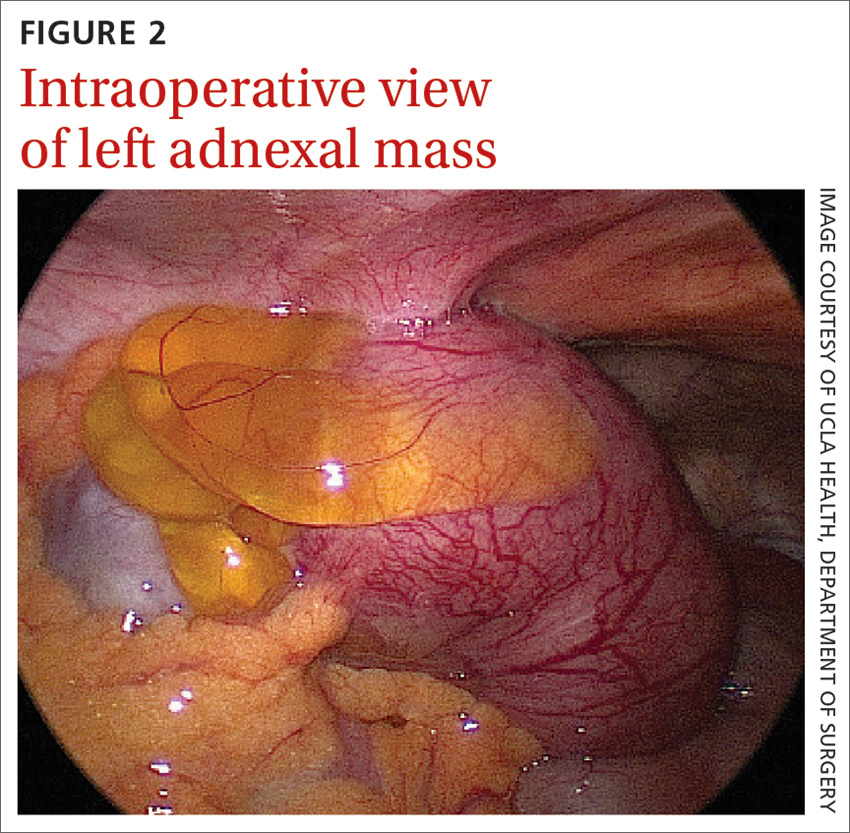
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
At the recommendation of her primary care physician, a 53-year-old perimenopausal woman sought care at the emergency department for the fever, abdominal pain, and pyuria that had persisted for 4 days despite outpatient treatment for pyelonephritis. On physical examination, she was febrile and tachycardic with abdominal tenderness of the left lower quadrant. Genitourinary examination revealed copious brown vaginal discharge, left adnexal tenderness, and no cervical motion tenderness.
Laboratory testing revealed leukocytosis but otherwise normal electrolytes, liver function tests, and lactate levels. Urine culture obtained when she presented to an urgent care facility 3 days earlier had been negative. Computed tomography (CT) was performed and was read by Radiology as “closed loop small bowel obstruction in the left lower abdomen” (FIGURE 1). The patient was taken emergently to the operating room where her entire length of bowel was run without any obstruction found. Instead, the surgeons identified a mass in the left iliac fossa originating from the left ovary and fallopian tube (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Pelvic inflammatory disease with tubo-ovarian abscess
The presence and location of this mass, paired with the patient’s symptoms, led to the diagnosis of pelvic inflammatory disease. PID is an acute infection of the upper genital tract in women thought to be due to ascending infection from the lower genital tract. The prevalence of PID in reproductive-aged women in the United States is estimated to be 4.4%.1
Diagnosis of PID in middle-aged women is a challenge given the broad differential diagnosis of nonspecific presenting symptoms, lower index of suspicion in this age group, and unknown exact incidence of PID in postmenopausal women. While delay in diagnosis of PID in women of reproductive age is associated with increased infertility and ectopic pregnancy,2 delay in diagnosis in postmenopausal women also poses serious potential complications such as tubo-ovarian abscess (TOA)—as was seen with this patient—and concurrent gynecologic malignancy found on pathology of TOA specimens.3,4
Risk factors for PID in the postmenopausal population include recent uterine instrumentation, history of prior PID, and structural abnormalities such as cervical stenosis, uterine anatomic abnormalities, or tubal disease. The microbiology of PID in postmenopausal women differs from that of women of reproductive age. While sexually transmitted pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis most commonly are implicated in PID among premenopausal patients, aerobic gram-negative bacteria including Escherichia coli and Klebsiella pneumoniae most frequently are associated in postmenopausal cases.
Differential diagnosis for abdominal pain is broad
The differential diagnosis for a patient with fever and abdominal pain includes PID, as well as the following:
Diverticulitis classically presents with left lower abdominal pain and a low-grade fever. Complications may include bowel obstruction, abscess, fistula, or perforation. Abdominal imaging such as a CT scan is required to establish the diagnosis.
Continue to: Urinary tract infection
Urinary tract infection should be suspected in a patient with dysuria, urinary frequency or urgency, and abdominal or flank pain. Urinalysis and culture should be performed and imaging may be considered for suspected obstruction, complication, or failure to improve on appropriate therapy.
Appendicitis may present as right lower quadrant pain with anorexia, fever, and nausea. Imaging studies such as CT or ultrasound can help support the diagnosis and rule out alternate etiologies of the presenting symptoms.
Ectopic pregnancy—while not considered in this case—should be suspected in a patient presenting with pelvic pain, missed menses or vaginal bleeding, and a positive pregnancy test. Further evaluation may be performed with a transvaginal ultrasound and serial measurement of serum quantitative human chorionic gonadotropin level.
Diagnosing PID is a clinical process
PID often is difficult to diagnose because of an absence of symptoms or the presence of symptoms that are subtle or nonspecific. Laparoscopy or endometrial biopsy can be useful but may not be justifiable due to their invasive nature when symptoms are mild or vague.5 Thus, a diagnosis of PID usually is based on clinical findings.
Clinical criteria to look for. Although PID commonly is attributed to N gonorrhoeae and C trachomatis, fewer than 50% of those with a diagnosis of acute PID test positive for either of these organisms.5 As such, the Centers for Disease Control and Prevention (CDC) 2015 Sexually Transmitted Diseases Treatment Guidelines recommend presumptive treatment for PID in women with pelvic or lower abdominal pain with 1 or more of the following clinical criteria: cervical motion tenderness, uterine tenderness, or adnexal tenderness.
Continue to: The following criteria...
The following criteria enhance specificity and support the diagnosis5:
- oral temperature > 101°F (> 38.3°C),
- abnormal cervical mucopurulent discharge or cervical friability,
- presence of “abundant numbers of white blood cells on saline microscopy of vaginal fluid,”
- elevated erythrocyte sedimentation rate (reference range, 0–20 mm/hr),
- elevated C-reactive protein (reference range, 0.08-3.1 mg/L), and
- laboratory documentation of cervical infection with N gonorrhoeae or C trachomatis.
The CDC also suggests that the most specific criteria for PID include5
- endometrial biopsy consistent with endometritis,
- imaging (transvaginal ultrasound or magnetic resonance imaging) demonstrating fluid-filled tubes, or
- laparoscopic findings consistent with PID.
Treatment of PID includes IV antibiotics
Due to the polymicrobial nature of PID, antibiotics should cover not only gonorrhea and chlamydia but also anaerobic pathogens. CDC guidelines recommend the following treatment5,6:
- intravenous (IV) cefotetan (2 g bid) plus doxycycline (100 mg PO or IV bid),
- IV cefoxitin (2 g qid) plus doxycycline (100 mg PO or IV bid), or
- IV clindamycin (900 mg tid) plus IV or intramuscular (IM) gentamicin loading dose (2 mg/kg) followed by a maintenance dose (1.5 mg/kg tid).
In mild-to-moderate PID cases deemed appropriate for outpatient therapy, the following regimens have been shown to have similar outcomes to IV therapy5,6:
- IM ceftriaxone (250 mg, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days,
- IM cefoxitin (2 g, single dose) and PO probenecid (1 g, single dose) plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days, or
- other parenteral third-generation cephalosporin plus PO doxycycline (100 mg bid) for 14 days with/without PO metronidazole (500 mg bid) for 14 days.
Management in older women may be more intensive
Due to the increased risk of malignancy in postmenopausal women with TOA, surgical intervention may be needed.3,4
Continue to: Our patient
Our patient underwent diagnostic laparoscopy, hysterectomy, left salpingo-oophorectomy, and right salpingectomy (with her right ovary left in place due to her perimenopausal status). Intraoperatively, she was found to have cervical stenosis. Postoperatively, she improved on IV cefoxitin (2 g qid) and IV doxycycline (100 mg bid), which was eventually transitioned to oral doxycycline (100 mg bid) and metronidazole (500 mg bid) on discharge.
Her final microbiology was negative for gonorrhea/chlamydia but the bacterial culture of peritoneal fluid grew E coli. Pathology was consistent with acute salpingitis, TOA, and acute cervicitis. She made a full recovery and is doing well.
CORRESPONDENCE
Catherine Peony Khoo, MD, 1920 Colorado Avenue, Santa Monica, CA 90404; Ckhoo@mednet.ucla.edu
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .
1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age—United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66:80-83.
2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185-192.
3. Jackson SL, Soper DE. Pelvic inflammatory disease in the postmenopausal woman. Infect Dis Obstet Gynecol. 1999;7:248-252.
4. Protopas AG, Diakomanolis ES, Milingos SD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114:203-209.
5. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
6. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937 .