User login
Postpartum psychosis: Protecting mother and infant
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
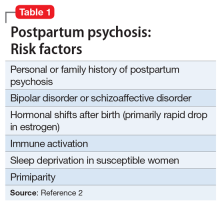
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
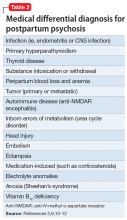
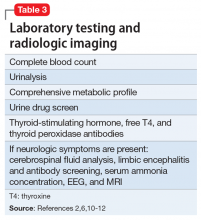
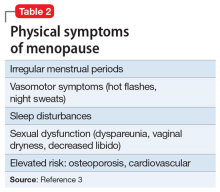
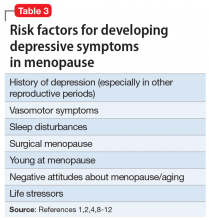
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
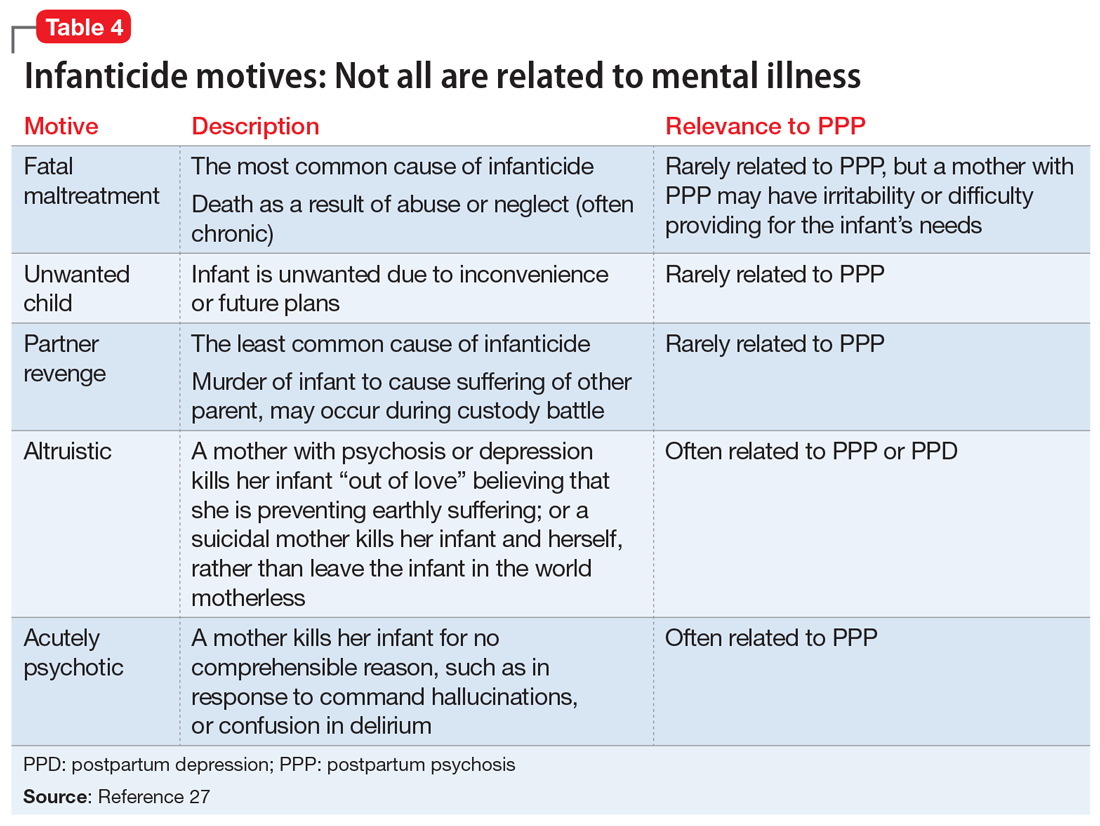
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
Psychiatric considerations in menopause
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
Mrs. J, age 49, presents to your psychiatric clinic. For the last few years, she has been experiencing night sweats and hot flashes, which she has attributed to being perimenopausal. Over the last year, she has noticed that her mood has declined; however, she has suffered several life events that she feels have contributed. Her mother was diagnosed with Alzheimer’s disease and had to move into a nursing home, which Mrs. J found very stressful. At the same time, her daughter left home for college, and her son is exploring his college options. Recently, Mrs. J has not been able to work due to her mood, and she is afraid she may lose her job as a consequence. She has struggled to talk to her husband about how she is feeling, and feels increasingly isolated. Over the last month, she has had increased problems sleeping and less energy; some days she struggles to get out of bed. She is finding it difficult to concentrate and is more forgetful. She has lost interest in her hobbies and is no longer meeting with her friends. She has no history of depression or anxiety, although she recalls feeling very low in mood for months after the birth of each of her children.
Are Mrs. J’s symptoms related to menopause or depression? What further investigations are necessary? Would you modify your treatment plan because of her menopausal status?
Women are at elevated risk of developing psychiatric symptoms and disorders throughout their reproductive lives, including during menopause. Menopause is a time of life transition, when women may experience multiple physical symptoms, including vasomotor symptoms (night sweats and hot flashes), sexual symptoms, and sleep difficulties. Depressive symptoms occur more frequently during menopause, and symptoms of schizophrenia may worsen.
Estrogen plays a role in mental illness throughout a woman’s life. In menopause, decreasing estrogen levels may correlate with increased mood symptoms, physical symptoms, and psychotic symptoms. As such, psychiatrists should consider whether collaboration regarding adjunctive hormone replacement therapy would be beneficial, and whether the benefits outweigh the potential risks. Otherwise, treatment of depression in menopause is similar to treatment outside of the menopausal transition, though serotonergic antidepressants may help target vasomotor symptoms while therapy may focus on role transition and loss. In this article, we review why women are at increased risk for mental illness during menopause, the role of estrogen, and treatment of mood and psychotic disorders during this phase of a woman’s life.
Increased vulnerability across the lifespan
Continued to: Why menopause?
Why menopause?
Perimenopausal mood disorders
However, one should keep in mind that new-onset mania in menopause is rare and should trigger a medical work-up and a dementia evaluation.13 Table 414 provides recommendations for evaluation of women undergoing menopause.
Menopause and serious mental illnesses
A study of 91 perimenopausal and postmenopausal women (age 45 to 55) who were diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or major depressive disorder (MDD) found that women with severe mental illness experienced significant vasomotor, physical, sexual, and psychosocial symptoms related to menopause.15 Furthermore, on 7 of 29 items on the Menopause Specific Quality of Life Scale, including hot flashes, women diagnosed with MDD reported problems significantly more often than women with other serious mental illnesses.15
Women with serious mental illness often have deficits in their knowledge about menopause.3 More than half of the 91 women in the study diagnosed with schizophrenia/schizoaffective disorder, bipolar disorder, or MDD felt more stressed related to menopause, and reported that menopause had a negative effect on their mental health.3 These women rated their top 5 symptoms potentially related to menopause as feeling depressed, anxious, or tired; lacking energy; and experiencing poor memory.3
Continued to: Role of estrogen on mood and psychosis
Role of estrogen on mood and psychosis
Women are at higher risk throughout their reproductive life than are men for MDD, anxiety disorders, and trauma-related disorders.12 Factors associated with depression during the menopause transition are reproductive hormonal changes (rise of follicle-stimulating hormone [FSH] and luteinizing hormone levels, and variability in estrogen [E2] and FSH levels); menopausal symptoms, particularly vasomotor symptoms; prior depression; psychosocial factors (adverse life events, financial strain, poor social supports); high body mass index, smoking, and poor physical health.6,7 Decreasing estrogen in the menopause transition may increase susceptibility to depression in some women.16 The Box17,18 provides more information on the relationship between estrogen and brain function.
Box
Estrogen and brain function
Numerous molecular and clinical studies have established the role of 17-beta estradiol in modulating brain functions via alterations in neurotransmission.17 Estrogen increases serotonin availability in the synapse by various pathways. It increases the rate of degradation of monoamine oxidase; monoamine oxidase enzymes are responsible for catabolizing serotonin, dopamine, and norepinephrine. Estrogen also increases tryptophan hydroxylase expression (rate-limiting enzyme in serotonin synthesis) and promotes intraneuronal serotonin transport in brain regions associated with affect regulation by increasing gene expression of the serotonin reuptake transporter. Studies have linked brain-derived neurotropic factor (BDNF) to increased serotonin turnover and proposed that estrogen may influence depression by increasing BDNF levels within the brain.18
Depressive disorders, including premenstrual dysphoric disorder, postpartum depression, and perimenopausal depression, have been linked to changes in hormonal status in women. Symptomatic menopause transition occurs in at least 20% of women, and a retrospective cohort study suggests that symptomatic menopause transition might increase the risk of new-onset depressive disorders, bipolar disorders, anxiety disorders, and sleep disorders.19 Symptomatic menopause transition also is a vulnerable time for relapse of MDD. Among women experiencing menopausal symptoms, including hot flashes, one-third also report depression—which correlates with a poorer quality of life, less work productivity, and greater use of health care services.9
Women who undergo surgical menopause are at greater risk for depression.8,10,11 This may be due to abrupt deprivation of estrogen—or related to a psychological reaction to the loss of fertility.
The observation that hormonal fluctuations related to women’s reproductive cycle have a significant impact on psychotic symptomatology has resulted in the “hypo-estrogenism hypothesis,” which proposes that gonadal dysfunction may increase vulnerability to schizophrenia, or that schizophrenia may lead to gonadal dysfunction.20 The “estrogen protection hypothesis” proposes that estrogen may protect women from schizophrenia, and may be a factor in the delayed onset of schizophrenia compared with men, less severe psychopathology, better outcomes, and premenstrual and postmenopausal deterioration in women. Many women of reproductive age with schizophrenia experience improvement in symptoms during the high estrogen phase of their menstrual cycle.
Pope et al21 have suggested that a hormone sensitivity syndrome may underlie why some women experience physical, psychological, and emotional symptoms at times of hormonal shifts such as menopause. This may represent a critical window of vulnerability, and also an opportunity to consider E2 as a therapeutic intervention.
Continued to: Treating mental illness in menopause
Treating mental illness in menopause
Changes to drug pharmacokinetics occur because some metabolising enzymes are estrogen-dependent and their levels decline after menopause, which leads to greater variability in drug response, particularly for oral medications. Other factors that can contribute to variability in medication response are polypharmacy, alcohol, illicit drugs, liver mass, smoking, caffeine, and nutritional intake.
While antidepressants are the first-line treatment for MDD and anxiety disorders, some patients remain unresponsive or inadequately responsive to currently available medications. In perimenopausal women with MDD, there may be an indication for adjunctive therapy with transdermal E2 in refractory cases; estrogen may augment the effects of selective serotonin reuptake inhibitor (SSRI) antidepressants as well as hasten the onset of antidepressant action.22 Estrogen also may be worth considering in women with mild depressive symptoms. For MDD, SSRIs plus estrogen may be more beneficial in improving mood than either agent alone. The effectiveness of E2 is less certain in postmenopausal depression.
Hormonal therapy for mental health disorders has equivocal evidence. The individual’s history and risk factors (eg, cardiovascular and osteoporosis risks) must be considered. A recent trial found that treatment with either venlafaxine or low-dose estrogen improved quality of life in menopausal women with vasomotor symptoms.23 Venlafaxine improved the psychosocial domain, while estrogen improved quality of life in other domains. Escitalopram, duloxetine, and citalopram have also been identified as having a possible positive impact on menopausal symptoms.22 SSRIs and serotonin-norepinephrine reuptake inhibitors may help reduce hot flashes and improve sleep.11
Regarding schizophrenia and estrogen, there may be improved symptoms during the high estrogen phase of the menstrual cycle, followed by a premenstrual aggravation of symptoms. Recall that women have a second peak of onset of schizophrenia after age 45, around the age of the onset of menopause.24 In a study of geropsychiatric hospital admissions, women were overrepresented among those with schizophrenia and schizoaffective disorder, compared with other psychiatric disorders.25 Postmenopausally, some women experience a decreased responsiveness to antipsychotics and worsening symptoms. In menopausal women with schizophrenia, check prolactin levels to help determine whether they are experiencing a natural menopause or medication-induced amenorrhea. Gender differences in pharmacotherapy responses and the decreasing response to antipsychotics in women older than age 50 have been observed26 and have led to exploration of the role of estrogen for treating schizophrenia in menopausal women. There have been contradictory results regarding use of estrogen as an adjunct to antipsychotics, with some reports finding this approach is effective and results in lower average doses of antipsychotics. Kulkarni et al27,28 have reported improvements in positive symptoms of treatment-resistant schizophrenia with transdermal use of E2, 200 mcg, as an adjunct to antipsychotics in women of childbearing age. However, they expressed caution regarding the health risks associated with prolonged use of E2. Long-term risks of high-dose estrogen therapies include thromboembolism, endometrial hyperplasia, and breast cancer, and individual factors should be considered before starting any form of hormone therapy. Selective estrogen receptor modulators (SERMs), such as raloxifene, which can cause activation of E2 receptors in a tissue-specific fashion and have less estrogen-related adverse effects, offer hope for future development in this field.27,28 While the use of adjunctive hormone therapy to manage psychotic symptoms in menopause is not routinely advised, the dosages of previously effective antipsychotics may need to be reviewed, or long-acting depot routes considered.29 Increased risk of prolonged QTc interval and tardive dyskinesia in geriatric women also should be considered in decisions regarding changes to antipsychotics or dosages.30
There are no guidelines regarding change in dosage of either individual antidepressants or antipsychotics in women at the time of menopause for managing pre-existing conditions. This may be due to the high variability in the effect of menopause on mental health and recognition that menopause is also a time for deterioration in physical health, as well as psychosocial changes for women, and thus other forms of intervention need to be considered.
Continued to: The biopsychosocial approach to treatment...
The biopsychosocial approach to treatment is particularly important in menopause.11 Common transitions in midlife include changes in relationships, employment, and financial status, and illness or death of family and friends.31 Therapy may focus on accepting a role transition and coping with loss of fertility. Cognitive-behavioral therapy may be helpful for menopausal symptoms, including hot flashes,4 as well as depressive symptoms.11
Although there are overlapping symptoms with both MDD and the perimenopause, these are typically restricted to impaired energy, sleep, and concentration, or changes in libido and weight.32 Therefore, it is vital to obtain a clear history and explore these symptoms in greater depth, as well as collect further information related to additional criteria such as appetite, agitation, feelings of worthlessness or guilt, and suicidal ideation.
Starting an antidepressant
On evaluation, Mrs. J discloses that she had experienced thoughts of wanting to end her life by overdose, although she had not acted on these thoughts. She appears subdued with poor eye contact, latency of response, and a slowed thought process. Mrs. J has blood tests to rule out thyroid abnormality or anemia. FSH and LH levels also are measured; these could provide a useful reference for later.
After a discussion with Mrs. J, she agrees to start an antidepressant. She also plans to speak to her gynecologist about the possibility of hormone replacement therapy. She is referred for psychotherapy to help support her with current life stressors. Mrs. J is started on escitalopram, 10 mg/d, and, after a month, she notices some improvement in her mood, psychomotor symptoms, sleep, and energy levels.
Bottom Line
Menopause is an important transition in our patients’ lives—both biologically and psychosocially. Women’s symptom patterns and medication needs may change during menopause.
Related Resource
- The North American Menopause Society. Depression & menopause. https://www.menopause.org/for-women/menopauseflashes/mental-health-at-menopause/depressionmenopause.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Raloxifene • Evista
Venlafaxine • Effexor
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.
1. Bromberger JT, Kravitz HM. Mood and menopause: findings from the study of women’s health across the nation (SWAN) over 10 years. Obstet Gynecol Clin North Am. 2011;38(3):609-625.
2. Almeida OP, Marsh K, Flicker L, et al. Depressive symptoms in midlife: the role of reproductive stage. Menopause. 2016;23(6):669-765.
3. Sajatovic M, Friedman SH, Schuermeyer IN, et al. Menopause knowledge and subjective experience among peri- and postmenopausal women with bipolar disorder, schizophrenia and major depression. J Nerv Ment Dis. 2006;194(3):173-178.
4. Ayers BN, Forshaw MJ, Hunter MS. The menopause. The Psychologist. 2011;24:348-353.
5. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888.
6. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
7. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382.
8. Georgakis MK, Thomopoulos TP, Diamantaras AA, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry 2016;73(2):139-149.
9. DiBonaventura MC, Wagner JS, Alvir J, et al. Depression, quality of life, work productivity, resource use, and costs among women experiencing menopause and hot flashes: a cross-sectional study [published online November 1, 2012]. Prim Care Companion CNS Disord. 2012;14(6): pii: PCC.12m01410. doi: 10.4088/PCC.12m01410.
10. Llaneza P, Garcia-Portilla MP, Llaneza-Suárez D, et al. Depressive disorders and the menopause transition. Maturitas. 2012;71(2):120-130.
11. Vivian-Taylor J, Hickey M. Menopause and depression: is there a link? Maturitas. 2014;79(2):142-146.
12. Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. 1: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2-3):85-96.
13. Friedman SH, Stankowski JE, Sajatovic M. Bipolar disorder in women. The Female Patient. 2007;32:15-24.
14. Soares C, Cohen L. The perimenopause, depressive disorders, and hormonal variability. Sao Paulo Med J. 2001;119(2):78-83.
15. Friedman SH, Sajatovic M, Schuermeyer IN, et al. Menopause-related quality of life in chronically mentally ill women. Int J Psychiatry Med. 2005;35(3):259-271.
16. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
17. Carretti N, Florio P, Bertolin A et al. Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod. 2005;20(6):1548-1553.
18. Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13-25.
19. Hu LY, Shen CC, Hung JH et al. Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore). 2016;95(6):e2800. doi: 10.1097/MD.0000000000002800.
20. Riecher-Rossler AW. Estrogens and schizophrenia. In: Bergemann N, Riecher-Rossler A, eds. Estrogen effects in psychiatric disorders. Wien, Austria: Springer-Verlag Wien; 2005:31-52.
21. Pope CJ, Oinonen K, Mazmanian D, et al. The hormonal sensitivity hypothesis: a review and new findings. Med Hypotheses. 2017;102:69-77.
22. Dennerstein L, Soares CN. The unique challenges of managing depression in mid-life women. World Psychiatry. 2008;7(3):137-142.
23. Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015;22(6):607-615.
24. Seeman MV. Psychosis in women: Consider midlife medical and psychological triggers. Current Psychiatry. 2010;9(2):64-68,75-76.
25. Sajatovic M, Friedman SH, Sabharwal J, et al. Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. J Geriatr Psychiatry Neurol. 2004;17(1):3-8.
26. Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
27. Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20(6):695-702.
28. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
29. Brzezinski A, Brzezinski-Sinai NA, Seeman MV. Treating schizophrenia during menopause. Menopause. 2017;24(5):582-588.
30. Lange B, Mueller JK, Leweke FM, et al. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. 2017;18(4):351-362.
31. Ballard KD, Kuh DJ, Wadsworth MEJ. The role of the menopause in women’s experiences of the ‘change of life.’ Sociology of Health & Illness. 2001;23(4):397-424.
32. Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. doi: 10.4088/PCC.08r00747blu.