User login
The art of delivering evidence-based dual antiplatelet therapy
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
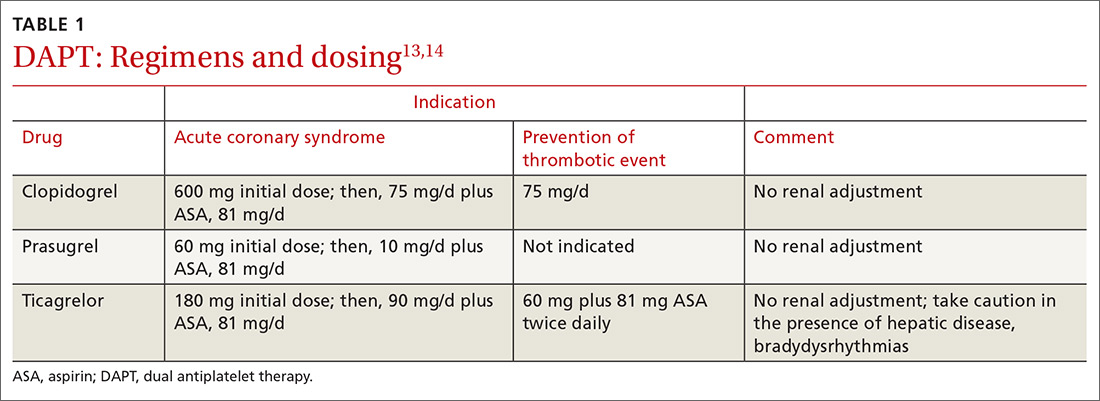
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
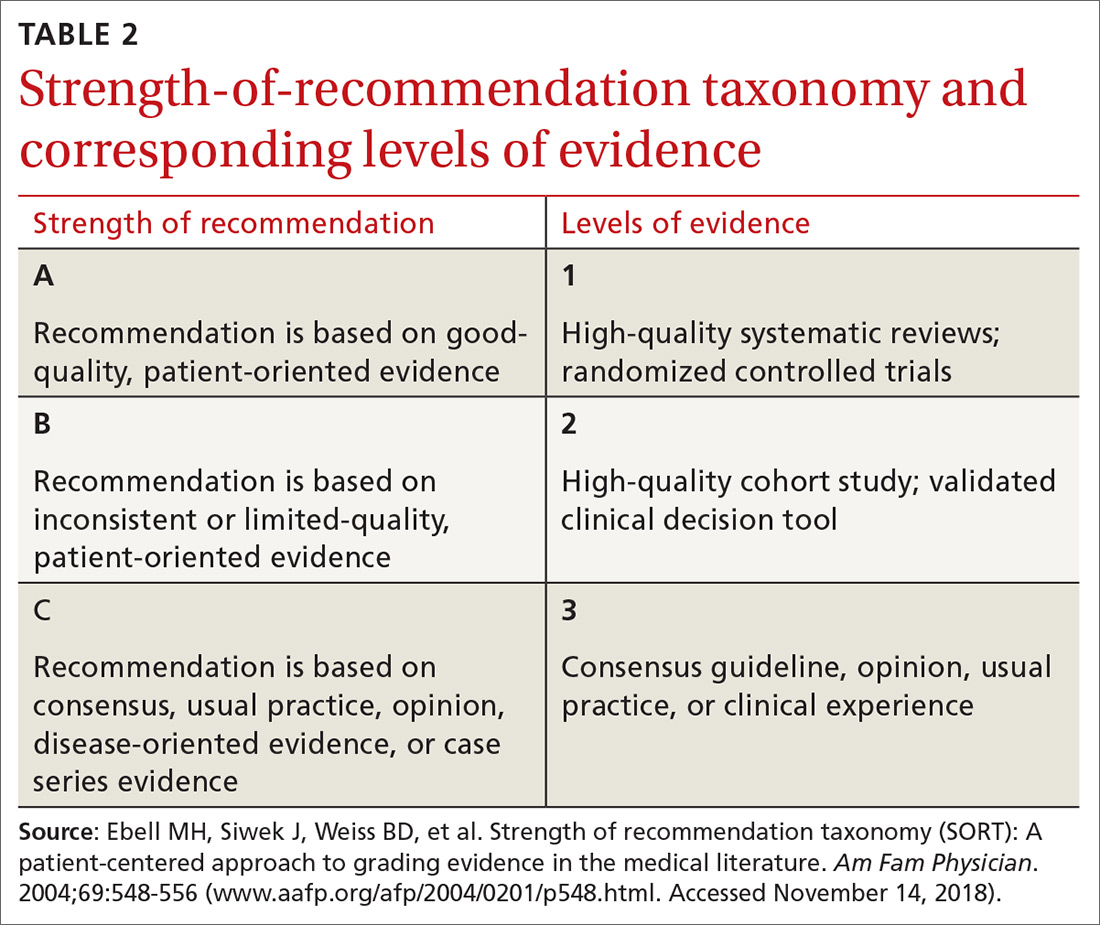
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
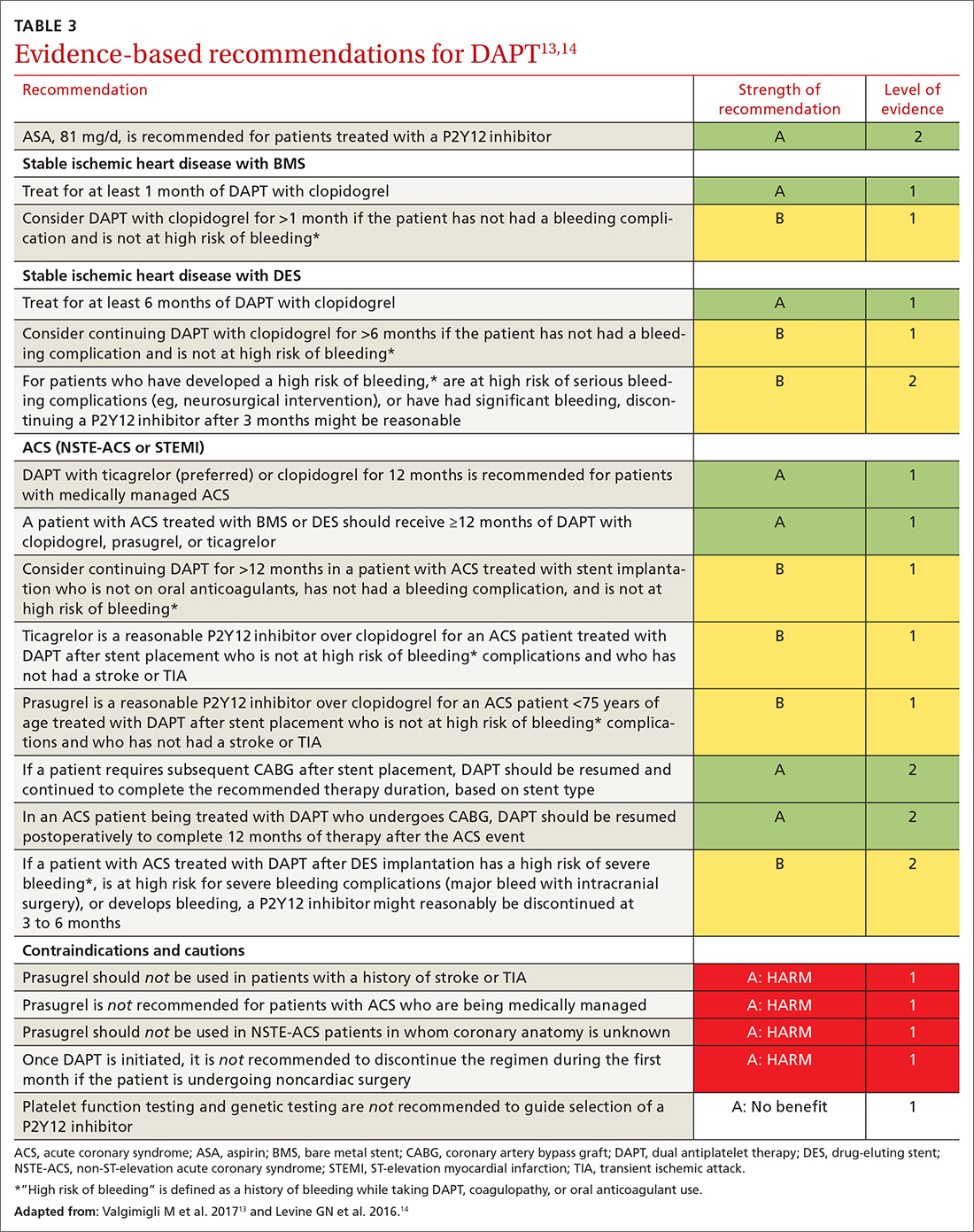
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
PRACTICE RECOMMENDATIONS
› Use a dual antiplatelet therapy (DAPT) risk calculator to encourage patient-centric decisions when presenting information to the health care team and the patient. B
› Consider the potential benefit of a shorter duration of DAPT for patients who 1) have prior bleeding complications or 2) are taking an oral anticoagulant, chronic corticosteroid, or nonsteroidal anti-inflammatory drug. B
› Continue aspirin use upon completion of DAPT or if a P2Y12 inhibitor is being held for surgery. A
› Reduce the risk of recurrent stroke in patients who have had a mild ischemic stroke or transient ischemic attack by providing DAPT for 21 to 28 days, followed by aspirin indefinitely—so long as treatment can begin within 24 hours of the event. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series