User login
Man, 25, With Sinus Pain, Sore Throat, and Rash
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
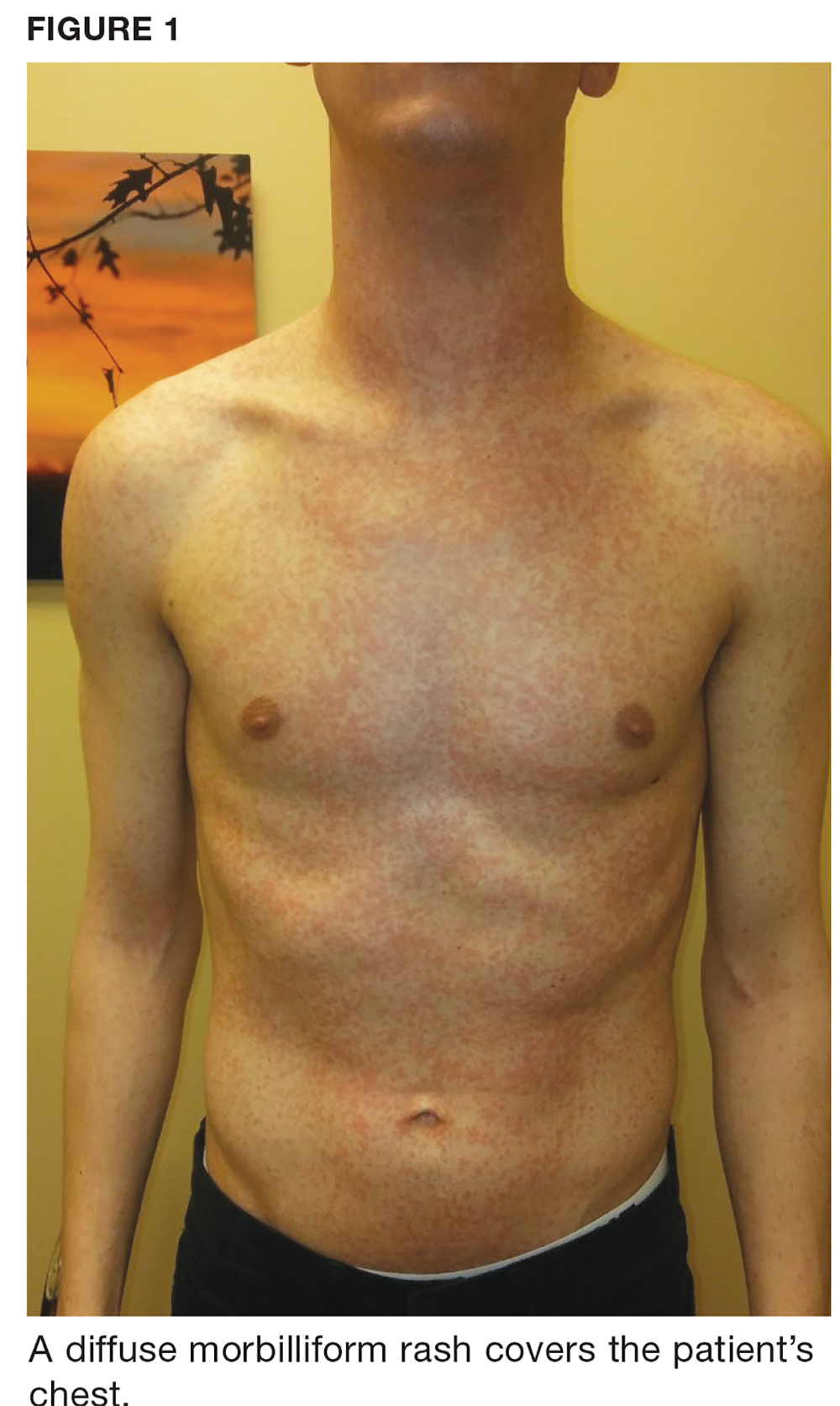
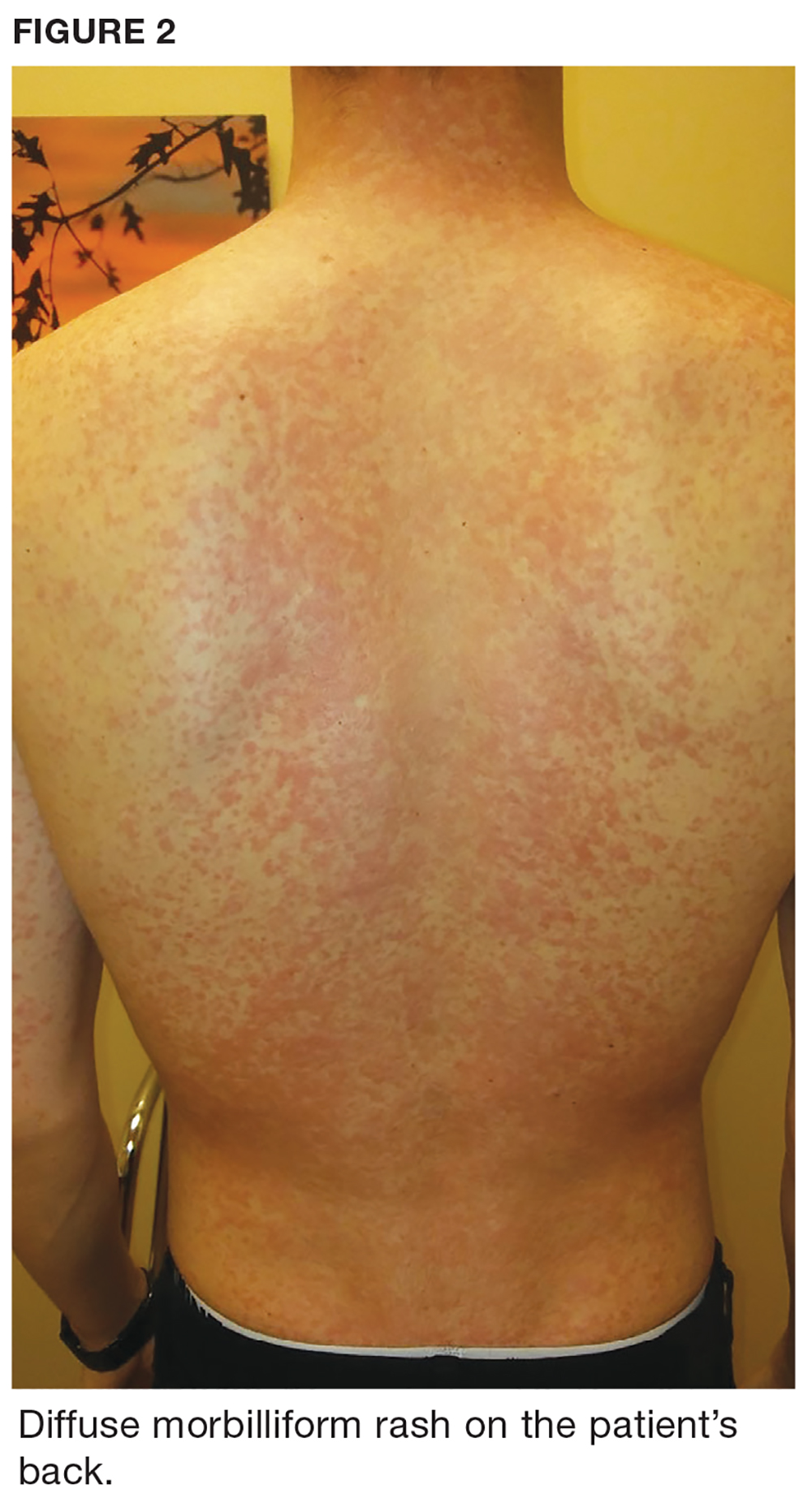
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
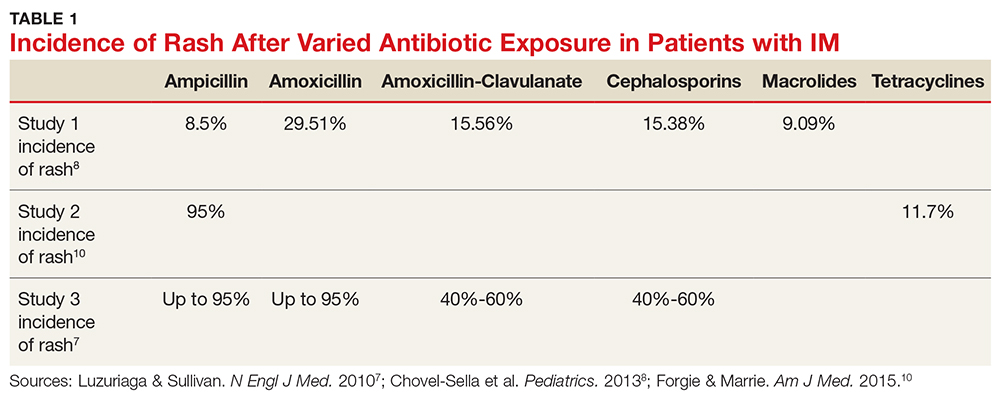
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
A 25-year-old white man presents to urgent care with a nine-day history of increasing sinus pressure, mild sore throat, dry cough, and low-grade fever. Physical exam of the ears, nose, throat, and chest is unremarkable, but the patient does display mild maxillary sinus tenderness. Sinus pain (and symptom duration) is the primary complaint. The patient was recently exposed to influenza B, but a rapid flu test is negative. A five-day course of amoxicillin-clavulanate is prescribed for a presumed diagnosis of bacterial sinusitis.
One week later, the patient returns with worsening sore throat and a morbilliform rash (see Figures 1 and 2), which covers the trunk, upper arms, and thighs. He has no known allergies to drugs, foods, or other environmental triggers. Examination reveals slightly tender, mobile anterior and posterior cervical lymphadenopathy, as well as bilateral tonsillar erythema and exudates, which were not present at the initial visit.
The rest of the exam is normal, and the patient’s sinus symptoms have resolved. Heterophile antibody testing yields positive results, suggesting infection with Epstein-Barr virus (EBV).
DISCUSSION
EBV is a pervasive herpesvirus that infects approximately 95% of adults worldwide.1 More than 90% of adults are seropositive for EBV antibodies by the age of 30.2 Although affected individuals are often asymptomatic, some patients develop symptoms of infectious mononucleosis (IM).2 An aminopenicillin rash can occur in patients with IM who are treated with amoxicillin or ampicillin, as was the case with this patient.
Incidence and pathophysiology
Infection with EBV most commonly occurs between the ages of 15 and 24.1,2 Infection before the age of 1 is rarely seen due to circulating maternal antibodies; incidence of IM in those younger than 1 or older than 30 is < 1 per 1,000 cases annually.2 The average annual incidence of infection is 0.5% in young adults (ages 15 and 24) but has been reported as high as 4.8%.2 About 10% to 20% of people who never knowingly come into contact with the virus will become infected annually; of those, up to 50% will develop IM.2 There are no known correlations in incidence based on sex or seasonal changes.2
Like all herpesviridae, EBV causes a latent infection that persists for a lifetime, specifically in replicating B lymphocytes.1 Saliva is the most common mode of EBV transmission, as viral shedding occurs in the throat and mouth.1,3 While the viral load in saliva is the highest during the first six months of infection, there are no clear data determining the risk for transmission throughout the course of asymptomatic shedding.4 There is a 30-to-50–day incubation period of EBV infection before a patient experiences symptoms of IM.1 During this period, B lymphocytes and epithelial cells (specifically in the tonsillar crypts) are believed to be the source of viral replication.1,3
Clinical presentation of IM
Common symptoms of IM include sore throat, fever, and fatigue. Approximately one in 13 patients ages 16 to 20 who present with a fever and sore throat will be diagnosed with IM.6 However, symptomatology alone is more sensitive than specific and is not sufficient to diagnose IM.6 Combined fatigue and pharyngitis is sensitive (81%-83%) but not specific, and posterior cervical lymphadenopathy increases the likelihood of IM (specificity, 87%).6
Continue to: The classic triad associated with IM includes...
The classic triad associated with IM includes fever, pharyngitis, and cervical lymphadenopathy, with morbilliform rash and palatal petechiae appearing less commonly (3%-15% and 25%, respectively).1,2,9 In affected patients, a transient truncal rash manifests within the first few days of disease onset.7 Tonsillar enlargement is also a common, but not specific, sign of acute IM.2 Splenomegaly is found in 15% to 65% of patients, typically developing within three weeks of disease onset.1,5,9
Hematologic complications occur in 25% to 50% of cases.5 Mild thrombocytopenia is common; however, more severe complications—such as hemolytic anemia, hemolytic-uremic syndrome, aplastic anemia, and disseminated intravascular coagulation—have also been associated with IM.5 Fulminant and potentially fatal complications are more common in immunocompromised patients.1,2
Pediatric and geriatric patients (those older than 65) may present with atypical signs and symptoms. For example, children are commonly asymptomatic or may present with a nonspecific viral illness.1 In addition, pediatric and elderly populations can develop elevated aminotransferase levels, and 26% of elderly patients present with jaundice (compared with 8% of young adults).2,3,7
Workup/differential diagnosis
Heterophile antibody testing is the most efficient and least expensive diagnostic test to confirm IM (sensitivity, 63%-84%; specificity, 84%-100%).2 Within the first week of IM, however, 25% of patients will produce a false-negative antibody test; a complete blood count (CBC) with differential and peripheral smear are appropriate follow-up tests.1,2,5 Detecting 10% or more atypical lymphocytes on a peripheral smear has a specificity of 95% and sensitivity of 61.3% for detecting IM, and a CBC with a lymphocyte count of less than 4,000 mm has a 99% negative predictive value.2 Viral capsid IgM testing can confirm the diagnosis of IM in an unclear clinical situation, such as a negative heterophile antibody test with an absolute lymphocyte count > 4,000 mm or in which 10% or more atypical lymphocytes were detected.2
Pharyngitis is caused by group A streptococci in 15% to 30% of children and 10% of adults worldwide, and 30% of patients with IM have a concomitant infection with group A streptococci.1,5 Because pharyngitis is a common presenting symptom of IM, rapid antigen strep test is appropriate when working up these patients.2 In addition, HIV, cytomegalovirus, human herpesvirus-6, and Toxoplasma gondii should be considered in the differential for patients with pharyngitis, fatigue, malaise, and lymphadenopathy—especially if the group A streptococci/EBV workup is negative.1,2,5
Continue to: EBV is also a known trigger of...
EBV is also a known trigger of hemophagocytic lymphohistocytosis (HLH). In a Japanese study, half of all HLH cases correlated with a primary infection of EBV.2,3,8 EBV is also the first confirmed oncogenic virus.3 EBV DNA in the plasma is now a tumor marker for nasopharyngeal carcinoma (sensitivity, 96%; specificity, 93%).8 Hodgkin lymphoma tumors are associated with EBV infection in 50% of cases.4 However, EBV seropositivity is ubiquitous (approximately 95%), while these correlated conditions are relatively uncommon; patient education on these issues is therefore not needed.
Treatment/complications
Aminopenicillin rash classically occurs in patients with IM who are treated with amoxicillin or ampicillin. These antibiotics are most commonly prescribed for suspected group A streptococci infection.7 Up to 95% of patients with IM who are exposed to these drugs develop this rash within two to 10 days of receiving the first dose of the antibiotic.9,10 Similar eruptions are often reported following administration of other penicillins, but not with the same frequency seen with ampicillin or amoxicillin (see Table 1).11
The mechanism of the aminopenicillin rash is not completely understood, but one theory is that the activated CD8+ cells react with the drug antigens and deposit in the skin.10 Another proposed mechanism is that antigens formed against activated polyclonal B cells create immune complexes with the drug, which then deposit in the skin.10
No known factors increase the incidence of this rash in patients after antibiotic exposure (eg, previous penicillin exposure, antibiotic dose or duration, patient age or ethnicity, atopic history).7 The rash generally resolves within a week after antibiotic discontinuation.7 Importantly, the development of a rash in a patient with EBV after administration of an aminopenicillin is not associated with an allergy nor is it a sign of an unfavorable reaction to such drugs in the future.12
The rash can be described as morbilliform or scarlatiniform and should be distinguished from the rash that acute IM can cause. Five percent of patients with an aminopenicillin rash will have an urticarial presentation, whereas 95% of patients have an exanthematous presentation.1,9,10 Although it can be quite difficult to distinguish one rash from the other, the aminopenicillin rash is more widespread than that associated with acute IM, covering extensor surfaces and spreading to the face, trunk, neck, mucous membranes, and sometimes the palms and soles.1,7,9,10 The rash caused by IM begins within the first few days of disease, whereas the aminopenicillin rash will manifest seven to 10 days after antibiotic exposure and is commonly pruritic.1 Each rash will last about one week.1
Continue to: CONCLUSION
CONCLUSION
The diagnosis of EBV can be challenging due to its similarity to group A streptococcal pharyngitis and other viral syndromes. In this case, the development of classic symptoms, along with the morbilliform eruption following administration of an aminopenicillin, was strongly suggestive of this diagnosis. This pairing of EBV infection and aminopenicillin rash does not indicate a penicillin allergy.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
1. Hall LD, Eminger LA, Hesterman KS, Heymann WR. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72(1):1-19.
2. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91(6): 372-376.
3. Tangye SG, Palendira U, Edwards ES. Human immunity against EBV—lessons from the clinic. J Exp Med. 2017; 214(2):269-283.
4. Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24:497-508.
5. Ebell MH, Call M, Shinholser J, Gardner J. Does this patient have infectious mononucleosis? The rational clinical examination systematic review. JAMA. 2016; 315(14):1502-1509.
6. Lernia VD, Mansouri Y. Epstein-Barr virus and skin manifestations in childhood. Int J Dermatol. 2013;52(10):1177-1184.
7. Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
8. Chovel-Sella A, Ben Tov A, Lahav A, et al. Incidence of rash after amoxicillin treatment in children with infectious mononucleosis. Pediatrics. 2013;131(5):e1424-e1427.
9. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513-522.
10. Forgie SED, Marrie TJ. Cutaneous eruptions associated with antimicrobials in patients with infectious mononucleosis. Am J Med. 2015;128(1):e1-e2.
11. Haverkos HW, Amsel Z, Drotman DP. Adverse virus-drug interactions. Rev Infect Dis. 1991;13(4):697-704.
12. Nazareth I, Mortimer P, McKendrick GD. Ampicillin sensitivity in infectious mononucleosis: temporary or permanent? Scand J Infect Dis. 1972;4(3):229-230.
Febrile, Immunocompromised Man With Rash
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
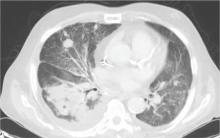
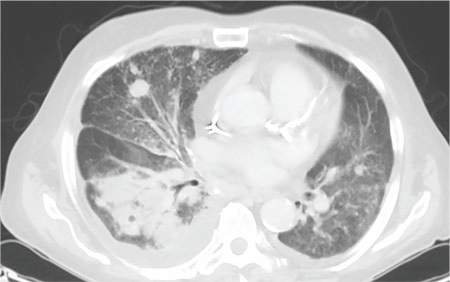
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
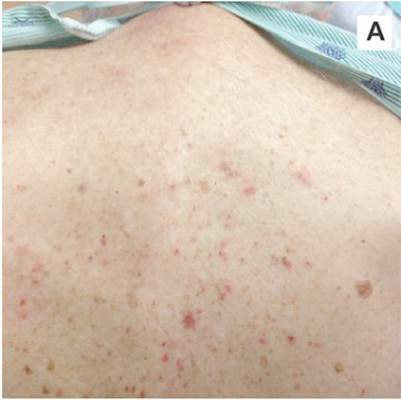 | 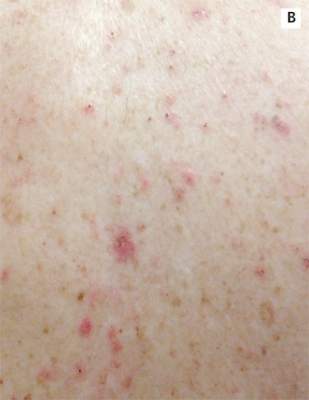 |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.