User login
When does asymptomatic aortic stenosis warrant surgery? Assessment techniques
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
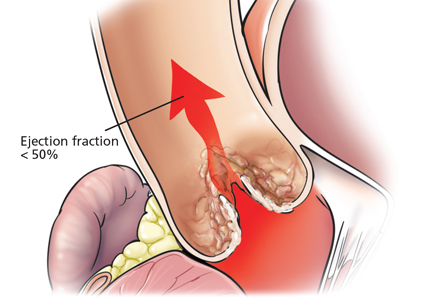
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
KEY POINTS
- Echocardiography is the best established and most important initial test in patients with suspected aortic stenosis.
- Traditional echocardiographic variables used in assessing aortic stenosis and the need for surgery are the pressure gradient across the valve, the velocity through the valve, the valve area, and the left ventricular ejection fraction.
- Aortic valve replacement is recommended for severe aortic stenosis if the patient has symptoms. It is also recommended if the left ventricular ejection fraction is less than 50%, if the patient is undergoing other cardiac surgery, or if symptoms arise on exercise stress testing.
- Novel assessment variables include left ventricular hypertrophy, left atrial size, B-type natriuretic peptide level, and global left ventricular longitudinal strain.