User login
Evidence in Prevention of Secondary Stroke
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
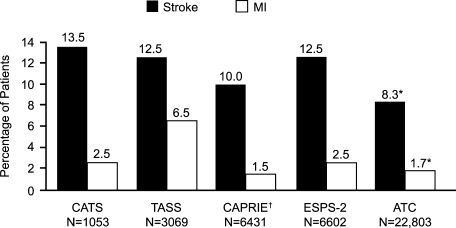
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
Stroke is a leading cause of disability and the third leading cause of death in the United States.1 Transient ischemic attack (TIA) carries a substantial short‐term risk for stroke.1 The risk of stroke following TIA ranges from 2% to 5% within 48 hours, is 10.5% within 90 days, and ranges from 24% to 29% within 5 years.24 Among the 780,000 new or recurrent strokes that occur each year, 180,000 are recurrent attacks.1, 5 Several evidence‐based guidelines for secondary prevention of stroke are available. To reduce variability in the assessment, diagnostic evaluation, and treatment of patients with TIA in actual clinical practice and to simplify the management of TIA or ischemic stroke, this article will review the available guidelines for secondary prevention of stroke and the data from clinical trials that support these guidelines.
PATHOPHYSIOLOGY AND SUBTYPES/CLASSIFICATION
Stroke is broadly classified as hemorrhagic or ischemic stroke. Hemorrhagic stroke, including intraparenchymal and subarachnoid hemorrhage, accounts for 13% of strokes and ischemic stroke for 87%.1 Ischemic stroke is caused by inadequate cerebral blood flow as a result of either stenosis or occlusion of the vessels supplying the brain.6 The average rate of cerebral blood flow is 50 mL/100 g a minute. Flow rates below 2025 mL/100 g a minute are usually associated with cerebral impairment, and rates below 10 mL/100 g a minute are associated with irreversible brain damage.
Approximately 20% of ischemic strokes are of cardioembolic origin; 25% are a result of atherosclerotic cerebrovascular disease; 20% are a result of penetrating artery disease (lacunes); 5% are due to other causes, such as hypercoagulable states, including protein S and C deficiency, sickle cell disease, and various types of vasculitis; and 30% are cryptogenic.7, 8 Cardioembolic stroke can be a manifestation of atrial fibrillation, valvular disease, ventricular thrombi, and other cardiac conditions.9 Large arteries, such as the carotid arteries and the proximal aorta, are a source of atherogenic emboli.10 Atherosclerotic plaques in the arteries may narrow the lumen of the blood vessel or produce emboli, which results in occlusion of the distal arteries, causing a stroke.
RISK FACTORS
Several risk factors, both nonmodifiable and modifiable, predispose individuals to stroke. Nonmodifiable risk factors include age, sex, race, and family or personal history of stroke or myocardial infarction (MI).1, 5 After the age of 55, the stroke rate doubles for every 10‐year increase in age.1 African Americans have a 50% greater risk of death due to stroke than whites.1 The appropriate management of modifiable risk factors can significantly reduce the risk of recurrent stroke and improve survival. The many modifiable factors include hypertension, heart disease, smoking, diabetes, atrial fibrillation, dyslipidemia, obesity, and alcohol abuse.1, 5 The mechanisms of how these factors increase the risk for stroke and management of these factors are discussed later in this article. It is important to educate individuals, particularly those who also have nonmodifiable risk factors, about modifiable risk factors in order to enable early and appropriate intervention.
DIAGNOSIS
Most patients with TIA are asymptomatic when they present to the emergency department (ED). The risk of stroke following an episode of TIA has been found to be 3.5% within 48 hours in a meta‐analysis based on a random effects model;11 therefore, it is critical to quickly identify patients with high short‐term risk for recurrent stroke.12 The ABCD2 score was recently validated in TIA patients to estimate the near‐term risk of completed stroke.13 Patients with a score of 03 on the ABCD2 are at low risk, those with a score of 4 or 5 are at moderate risk, and those with a score 6 or 7 are at severe risk for recurrent stroke (Table 1).13 Risk scores, although highly predictive, should complement clinical judgment in the assessment of individual stroke risk.
| Risk factors | Points |
|---|---|
| |
| AAge > 60 years | 1 |
| BBlood pressure | |
| Systolic 140 mm Hg | 1 |
| Diastolic 90 mm Hg | 1 |
| CClinical features | |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| DDuration of symptoms | |
| 1059 minutes | 1 |
| 60 minutes | 2 |
| DDiabetes | 1 |
Currently, there are no specific guidelines for the diagnostic evaluation of patients with suspected TIA. However, the following approach, including elements of acute evaluation for both stroke and TIA as well as risk factor identification that may aid in choosing specifics of secondary prevention, may be adopted in the management of patients with TIA (Table 2).14, 15
| Diagnostic test | Indication |
|---|---|
| |
| Acute phase | |
| CT brain (noncontrast) | Rule out intracerebral or subarachnoid hemorrhage and may show early signs of stroke; if clinically suspected subarachnoid hemorrhage, lumbar puncture should be performed |
| CT angiogram with CT perfusion | Visualize occluded vessel and identify infarcted versus at‐risk tissue |
| Chest radiograph | Potentially identify aortic aneurysm or lung masses prone to hemorrhage |
| Finger stick (glucometer testing) | Rule out hypoglycemia as etiology; follow‐up glucose screening may identify diabetes as a risk factor |
| Basic metabolic panel | Rule out metabolic problems leading to symptomatology and renal disease, which may prevent contrast imaging |
| Coagulation profiles | Rule out preexisting coagulopathy that would make patient prone to hemorrhage or ineligible for some therapies, including tissue plasminogen activator |
| Stool guaiac | Rule out gastrointestinal bleed, which may make patient ineligible for some therapies |
| Electrocardiogram | Rule out concurrent myocardial infarction or cardiac arrhythmia |
| Postacute phase | |
| MRI/MRA: diffusion and perfusion studies | Quantify region of infarcted tissue and affected arterymay be useful in acute phase if available on an expedited basis |
| Transthoracic/transesophageal echocardiogram | Rule out cardioembolic stroke etiology (ie, mural thrombus, patent foramen ovale, valvular disease) |
| Carotid duplex | Rule out carotid stenosis as stroke risk factor (secondary prevention) |
| Lipid profile | Rule out hyperlipidemia as stroke risk factor (secondary prevention) |
| Blood tests: antinuclear antibodies, rapid plasma reagin test, thyroid panel, antiphospholipid antibodies; other tests for hypercoagulability | Rule out other reasons for hypercoagulable state in the appropriate patient population |
A computed tomography (CT) scan of the head or magnetic resonance imaging (MRI) of the brain should be performed as soon as possible to distinguish between ischemic and hemorrhagic stroke, eliminate other pathologies that mimic TIA or stroke, and guide selection of the appropriate treatment approach. CT scanning is often the best initial imaging choice because it reliably excludes intracranial hemorrhage and is rapidly available in most settings. For those for whom the diagnosis is uncertain, diffusion‐weighted MRI may be more helpful. Because of the time issues surrounding the use of tissue plasminogen activator, waiting for an MRI may not always be the best choice, although some institutions are now able to provide quick access to MRI imaging. Imaging can detect silent cerebral infarcts associated with an increased risk of stroke. In patients with previous TIA and/or stroke, MRI is more sensitive than CT in detecting small, old infarcts (although most are seen on CT) and in visualizing the posterior fossa (cerebellum and brain stem).12
Holter electrocardiography or inpatient telemetry monitoring can be performed to identify atrial fibrillation, a known risk factor for stroke or TIA.16 Transesophageal echocardiography (TEE) has been reported to be more sensitive than transthoracic echocardiography (TTE) for detecting cardioembolic sources of TIA or ischemic stroke across multiple age groups.17 TEE has several advantages over TTE, such as the creation of clearer images of the aorta, the pulmonary artery, valves of the heart, both atria, the atrial septum, and the left atrial appendage.
Cerebral angiography is indicated in several instances, including in children or young patients with ischemic stroke because vascular abnormalities and cerebral vasculitis are relatively more common causes in patients in these age groups.18 Furthermore, in centers in which intra‐arterial procedures are frequently performed, angiography is indicated to confirm the suspicion of posterior circulation vessel (ie, vertebral or basilar artery) occlusion prior to intervention. Angiography has the highest diagnostic validity compared with other noninvasive techniques and may be indicated if cerebral vasculitis or nonatherosclerotic disease of extracranial arteries (eg, dissections, vascular malformations) is suspected. Angiography of intracranial vessels is the gold standard for the study of cerebral aneurysms and is recommended in patients with subarachnoid hemorrhage, but there is evidence that magnetic resonance angiography (MRA) and digital subtraction angiography have better discriminatory ability in the 70%99% range of stenosis compared with duplex ultrasonography (DUS) for determining candidacy for carotid endarterectomy (CEA) or stenting.19, 20
The MRA and CT angiography (CTA) are generally used to visualize the intracranial and extracranialboth anterior and posteriorcerebral circulation. The use of MRA or CTA to image cerebral circulation has generally supplanted the use of carotid and transcranial ultrasonography and obviated the need for catheter angiography in investigating the etiology of most ischemic strokes and TIAs. The degree of carotid stenosis should be primarily estimated using noninvasive techniques (DUS, MRA, CTA).21 Duplex ultrasonography is recommended after CEA 6 months and every 1 2 years after the procedure in order to monitor recurrent stenosis.22 Angiography should be performed when the results of noninvasive examinations are discordant; when significant atherosclerotic disease of intracranial arteries is suspected, especially in vertebrobasilar arteries; or when MRA or CT angiography provides technically poor images.23
Transcranial Doppler ultrasonography and color Doppler ultrasound (TCD) are used to evaluate the intracranial vessels and may provide additional information on patency of cerebral vessels, recanalization, and collateral pathways. Compared with the gold standard of conventional angiography, TCD has a positive predictive value of 36% and a negative predictive value of 86% for a diagnosis of intracranial stenosis.24 This technique also can be used as a complementary examination in patients undergoing CEA in order to aid in preoperative evaluation and intraoperative monitoring of blood flow in the territory of the operated artery.12
TREATMENT
The management of ischemic stroke or TIA includes lifestyle modifications, reduction of modifiable risk factors, and appropriate surgical and medical intervention.12
Lifestyle Modifications
There is strong evidence for smoking as an independent risk factor for ischemic stroke, irrespective of age, sex, or ethnic background.25 Among smokers, the risk for ischemic stroke is twice that of nonsmokers.26 All patients with previous ischemic stroke or TIA are strongly encouraged not to smoke and to avoid smoke in their environments as much as possible. These patients are also recommended to obtain counseling and smoking cessation medications as needed; these interventions should be started at the time of hospital admission.
The relationship of alcohol consumption to cardiovascular risk is controversial because most studies suggest a J‐shaped association between alcohol and ischemic stroke: a protective effect forthose who consume light‐to‐moderate amounts of alcohol (<60 g ethanol/day)27 and elevated stroke risk for heavy drinkers.28 The protective effect of moderate drinking may be related to an increase in high‐density lipoprotein cholesterol,29, 30 reduced platelet aggregation,31 and lower plasma fibrinogen concentration.32 In contrast, heavy drinking can lead to alcohol‐induced hypertension,33 a hypercoagulable state, reduced cerebral blood flow, and atrial fibrillation. Patients with prior ischemic stroke or TIA who are heavy drinkers are recommended to reduce or eliminate alcohol consumption.34
Obesity (body mass index [BMI] > 30 kg/m2) is an independent risk factor for coronary heart disease and premature mortality.1 Obesity is also associated with several other risk factors, such as hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.35 Indeed, obesity is often a symptom of metabolic syndrome, a combination of medical disorders that increases a person's risk for cardiovascular disease and diabetes (the International Diabetes Federation consensus worldwide definition of metabolic syndrome). All ischemic stroke or TIA patients who are overweight should maintain a goal BMI of 18.524.9 kg/m2 and a waist circumference of less than 35 inches, if female, or less than 40 inches, if male, because abdominal obesity is more related to stroke risk.36 Clinicians should recommend caloric restriction as the cornerstone of weight loss along with diets low in fat and cholesterol, increased physical activity, and behavioral counseling. A recent retrospective review suggests that moderately or highly active individuals have a lower risk of stroke or mortality than those whose physical activity is low.37 Physical activity exerts its beneficial effects by lowering blood pressure and weight, enhancing vasodilation, improving glucose tolerance, and promoting cardiovascular health.
Management of Modifiable Risk Factors
Hypertension
An estimated 73 million Americans have hypertension.1 Meta‐analyses of randomized trials confirm that lowering blood pressure is associated with a 30%40% reduction in stroke risk.38, 39 Because hypertension is a risk factor for many cardiovascular and cerebrovascular conditions, detailed evidence‐based recommendations for blood pressure screening and treatment of individuals with hypertension are summarized in the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on the primary prevention of ischemic stroke.40 More detailed information is available in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.41 Antihypertensive treatment is recommended for the prevention of recurrent stroke and other vascular events in individuals with ischemic stroke who are beyond the period immediately after an ischemic stroke regardless of whether they have a history of hypertension. Average blood pressure reduction of 10/5 mm Hg or maintenance of normal blood pressure (<120/80 mm Hg) is associated with benefits via diet, exercise, or medication.42 In a meta‐analysis of 7 trials that included a total of 15,527 patients, treatment with antihypertensive agents was associated with a 24% reduction in total stroke (P = .005), a 21% reduction in nonfatal stroke (P = .01), and a nonsignificant 24% reduction in fatal stroke (P = .08).42 The choice of specific drugs, discussed in the antihypertensive section of this article, and the target blood pressure should be individualized.
Diabetes
Diabetes affects 8% of the adult U.S. population, and several studies have reported that 15%33% of patients with ischemic stroke have diabetes.4345 The prevalence of diagnosed diabetes is projected to rise to 29 million by 2050 from the current 11 million, an increase of 165%.46 Diabetes is a critical independent risk factor for ischemic stroke. Rigorous control of blood pressure and lipid level is recommended in patients with diabetes, as well as in patients with hypertension and/or elevated cholesterol.5 Several agents used to treat diabetes, such as metformin and pioglitazone, improve glucose and lipid metabolism and exert antiatherogenic effects, aiding in the prevention of atherosclerosis.47 Glycemic control is recommended for patients with diabetes in order to prevent stroke and cardiovascular disease, but data are limited. Randomized trial data have shown that continual reduction of vascular events is correlated with control of glucose to normal levels.48
Elevated Cholesterol
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines recommend that lifestyle modification, diet, and medications be used to manage ischemic stroke or TIA patients with elevated cholesterol, comorbid coronary artery disease, or evidence of atherosclerosis. The target goal for those with coronary heart disease or symptomatic atherosclerosis is low‐density lipoprotein (LDL) cholesterol below 100 mg/dL.49 The 2004 update to the NCEP guidelines proposed an LDL cholesterol target below 70 mg/dL in very high‐risk patients or in those with established CHD plus multiple major risk factors (especially diabetes), severe and poorly controlled risk factors (especially continued cigarette smoking), multiple risk factors of the metabolic syndrome (especially high triglycerides [ 200 mg/dL] plus nonhigh‐density lipoprotein [HDL] cholesterol 130 mg/dL with low HDL‐C [<40 mg/dL]), or patients with acute coronary syndromes.50
Medical Treatment
Antiplatelet therapy is the cornerstone of secondary prevention of stroke.51 Four antiplatelet drugs are availableaspirin, clopidogrel, dipyridamole, and ticlopidinethat are approved by the U.S. Food and Drug Administration for secondary prevention of stroke. The following sections review the evidence for the efficacy and safety of these drugs for the secondary prevention of stroke (Table 3).5268 The role of anticoagulation for secondary prevention of noncardioembolic stroke is also discussed (Table 4).6971
| Study | Population | Treatment | Duration | Risk reduction | Outcome |
|---|---|---|---|---|---|
| |||||
| ATC52 | 70,000 High‐risk patients | Antiplatelet (mostly aspirin 75325 mg/day), placebo | >1 month | RRR, 25% vs. placebo; ARR, 3.3% | Vascular events (nonfatal MI, nonfatal stroke, vascular death) |
| IST53 | 19,435 Patients with acute ischemic stroke | Heparin 5000 or 12,500 U/day, aspirin 300 mg/day, heparin + aspirin, placebo | 14 days | Risk of ischemic stroke, 2.8% with aspirin vs. 3.9% in nonaspirin groups | Nonfatal stroke |
| CAPRIE56 | 19,185 Patients with recent ischemic stroke, MI, or atherosclerotic PAD | Clopidogrel 75 mg/day, aspirin 325 mg/day | 13 years (mean, 1.91 years) | RRR, 8.7% clopidogrel vs. aspirin; ARR, 0.5% with clopidogrel | MI, stroke, or vascular death |
| MATCH58 | 7599 Patients with recent ischemic stroke or TIA plus 1 additional vascular risk factor | Clopidogrel 75 mg/day, clopidogrel + aspirin 75 mg/day | 1.5 years | RRR, 6.4% combination vs. aspirin (NS) | Ischemic stroke, MI, vascular death, hospitalization for ischemic event |
| CHARISMA59 | 15,603 Patients with established cardiovascular disease or multiple risk factors | Clopidogrel 75 mg/day + aspirin 75162 mg/day, aspirin alone | 2 years | RRR, 7% for combination vs. aspirin | MI, ischemic stroke, vascular death |
| ESPS‐261 | 6602 Patients with TIA or stroke in previous 3 months | Aspirin 50 mg/day, dipyridamole 200 mg twice daily, aspirin + dipyridamole, placebo | 2 years | RRR, 37% combination vs. placebo; ARR, 3.4% combination vs. aspirin | Secondary stroke |
| ESPRIT65 | 2739 Patients with TIA or minor ischemic stroke | Aspirin (30325 mg/day), aspirin + dipyridamole (200 mg twice daily), oral anticoagulants | 5 years | RRR, 20% combination vs. aspirin; ARR, 1% per year combination vs. aspirin | Vascular death, nonfatal MI, nonfatal stroke |
| Study | Key efficacy results | Key safety results |
|---|---|---|
| ||
| WARSS70 | No difference between warfarin and aspirin in prevention of recurrent ischemic stroke, death, or rate of major hemorrhage | Although safety profile of warfarin was similar to aspirin in this study, there is potential increased risk in a community setting |
| WASID71 | Warfarin provided no additional benefit over high‐dose aspirin (1300 mg/day) for prevention of recurrent stroke or death | Warfarin was associated with significantly higher rates of adverse events |
| ESPRIT69 | Oral anticoagulants did not provide additional benefit over aspirin for prevention of TIA or minor stroke of arterial origin | Oral anticoagulants were associated with increased incidence of bleeding complications |
Aspirin
The Antiplatelet Trialists' Collaboration (ATC) determined the effect of prolonged antiplatelet therapy on vascular events (nonfatal MI, nonfatal stroke, or vascular death) in various patient groups.52 This retrospective analysis included about 70,000 high‐risk patients and 30,000 low‐risk patients from 145 randomized trials that compared prolonged antiplatelet therapy versus control and about 10,000 patients from 29 randomized trials that directly compared different antiplatelet regimens. Overall, the typical reduction in risk for these vascular events was 25% (SD 2%) with antiplatelet therapy compared with placebo (P < .001). The most commonly used antiplatelet regimen was medium‐dose aspirin (75325 mg/day). The number needed to treat (NNT) was 30 (absolute risk reduction [ARR], 3.3%) for 2.5 years for prevention of vascular events with aspirin.
The International Stroke Trial was a large, randomized, open‐label trial of up to 14 days of antithrombotic therapy immediately following the onset of stroke.53 In this trial, 19,435 patients were randomly assigned to receive unfractionated heparin (5000 or 12,500 IU twice daily) or aspirin (300 mg/day), alone or in combination, or placebo. The primary outcomes were death within 14 days and death or dependency at 6 months. Heparin treatment was not associated with a significant reduction in deaths within 14 days (876 [9.0%] vs. 905 [9.3%] with placebo) or rate of death or dependency at 6 months (62.9% in both groups). Heparin treatment was associated with an increase in the rate of hemorrhagic stroke and a significant excess of 9 (SD 1) transfused or fatal extracranial bleeds per 1000. Aspirin was not associated with a significant reduction in death within 14 days (872 [9.0%] vs. 909 [9.4%]; however, at 6 months, there was a nonsignificant trend toward a smaller proportion of deaths or dependency in those receiving aspirin (62.2% vs. 63.5%; P = .07), a difference of 13 (SD 7) deaths per 1000. Patients receiving aspirin had significantly fewer recurrent ischemic strokes within 14 days (2.8% vs. 3.9%; P < .001) with no significant increase in hemorrhagic strokes (0.9% vs. 0.8%), resulting in a significant reduction in the incidence of death or nonfatal recurrent stroke (11.3% vs. 12.4%, P = .02). Aspirin alone was associated with an excess of 2 (SD 1) transfused or fatal extracranial bleeds per 1000. These data suggest that aspirin should be started immediately after an ischemic stroke. The NNT for 14 days was 91 to prevent 1 nonfatal stroke.53
The efficacy of a lower dose of aspirin (30 mg/day) was compared with that of aspirin 238 mg/day by the Dutch TIA Trial Study Group. The results showed that the lower dose of aspirin was as effective as the higher dose in the prevention of a recurrent vascular event, and patients taking the lower dose had fewer adverse events.54
However, aspirin resistance is an issue of ongoing research and debate. It is one of several explanations for the limited efficacy of aspirin in the stroke population. Results of one study showed that resistance to aspirin in platelet function was not uncommon, as measured by platelet aggregation 24 hours and 3, 6, and 12 months following initiation of aspirin therapy.55
Clopidogrel
The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg/day) and aspirin (325 mg/day) in reducing the risk of the composite outcome of ischemic stroke, MI, or vascular death.56 In this study, 19,185 patients with atherosclerotic vascular disease (recent ischemic stroke, recent MI, or symptomatic peripheral arterial disease) were followed up for 1.91 years. Clopidogrel was associated with a 5.32% risk of the primary composite outcome compared with 5.83% with aspirin (relative risk reduction [RRR], 8.7%; 95% CI, 0.3%16.5%; P = .043). The NNT was 196 (ARR, 0.51%; 95% CI, 1024188; P = .043) for 1 year with clopidogrel instead of aspirin to prevent 1 patient from having a stroke, MI, or vascular death.56 Both treatments were associated with a similar safety profile. In a prespecified subgroup analysis among patients with a previous stroke, the risk reduction with clopidogrel was nonsignificant. However, in a post hoc analysis of patients with diabetes enrolled in the CAPRIE trial (n = 3866), clopidogrel was associated with a greater benefit than aspirin (ARR, 2.1%; P = .042) compared with no benefit in nondiabetic patients.57
In the Management of Atherothrombosis with Clopidogrel in High‐Risk Patients with TIA or Stroke (MATCH) trial, 7599 patients with a prior stroke or TIA plus additional risk factors received clopidogrel 75 mg/day or combination therapy of clopidogrel 75 mg/day plus aspirin 75 mg/day.58 The primary outcome was the composite of ischemic stroke, MI, vascular death, or rehospitalization secondary to ischemic events. There was no significant benefit of combination therapy compared with clopidogrel alone in reducing the primary outcome (RRR, 6.4%; 95% CI, 4.6%16.3%; ARR, 1%; 95% CI, 0.6%2.7%) or any of the secondary outcomes. The risk of major hemorrhage was significantly increased in the combination group compared with clopidogrel alone, with a significant 1.3% absolute increase in life‐threatening bleeding (95% CI, 0.6%1.9%). Although clopidogrel plus aspirin is recommended over aspirin for acute coronary syndromes, with most guidelines advocating up to 12 months of treatment, the results of the MATCH trial do not suggest a similar risk reduction for stroke patients.58
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial investigated the efficacy of dual antiplatelet therapy with clopidogrel (75 mg/day) plus low‐dose aspirin (75162 mg/day) versus low‐dose aspirin alone in reducing subsequent stroke and MI and death from cardiovascular causes in 15,603 men and women with clinically evident cardiovascular disease or multiple cardiovascular risk factors.59 At the end of follow‐up, there was no significant difference between treatments in the primary efficacy outcome (6.6% with clopidogrel plus aspirin vs. 7.3% with aspirin alone; relative risk [RR], 0.93; 95% CI, 0.831.05; P = .22). The combination was associated with a greater incidence of gastrointestinal bleeding (number needed to harm, 88; 95% CI, 59‐170) over 28 months. There was a nonsignificant increase in the risk of severe bleeding with clopidogrel in combination with aspirin compared with aspirin alone (RR, 1.2; 95% CI, 0.911.59; P = .20). Among patients with multiple risk factors (but no clinically evident cardiovascular disease), cardiovascular mortality was significantly higher with clopidogrel plus aspirin (3.9%) versus aspirin alone (2.2%; P = .01).59
Recently, a post hoc analysis of data from CHARISMA was performed to assess the possible benefit of dual antiplatelet therapy in a subgroup of patients (n = 9478) with a documented history of MI, ischemic stroke, or symptomatic peripheral arterial disease.60 In this subgroup, the rate of cardiovascular death, MI, or stroke was significantly lower in the clopidogrel‐plus‐aspirin group compared with aspirin alone (7.3% versus 8.8%; hazard ratio [HR], 0.83; 95% CI, 0.720.96; P = .01). There was no significant difference in severe bleeding between the clopidogrel‐plus‐aspirin and aspirin‐alone groups in this subpopulation (1.7% vs. 1.5%; HR, 1.12; 95% CI, 0.811.53; P = .50). However, there was a significantly higher increase in moderate bleeding with clopidogrel plus aspirin compared with aspirin alone (2.0% versus 1.3%; HR, 1.60; 95% CI, 1.162.20; P = .004). These data from the post hoc subanalysis suggest that a large proportion of patients with documented prior MI, ischemic stroke, or symptomatic peripheral artery disease may derive significant benefit from dual antiplatelet therapy with clopidogrel plus aspirin.60 These observations do not support the observations in the MATCH trial; therefore, additional studies are required to validate these findings.
Aspirin Plus Extended‐Release Dipyridamole
In the Second European Stroke Prevention Study (ESPS‐2), 6602 patients with prior stroke or TIA were assigned to low‐dose aspirin (25 mg twice daily) plus extended‐release dipyridamole (ER‐DP; 200 mg twice daily), aspirin alone, ER‐DP alone, or placebo.61 The extended‐release formulation of dipyridamole provided the benefits of continuous absorption and steady serum levels, resulting in a more consistent response in a narrow therapeutic index, especially in the elderly.62 The relative risk of stroke was reduced by 37% with the combination treatment versus 18% with low‐dose aspirin alone or 16% with dipyridamole alone. The combination treatment was also associated with a significant reduction (36%) in the risk of TIA compared with placebo (P < .001).61 Thus, significantly greater protective effects were seen with the combination therapy. Gastrointestinal bleeding was more common in patients receiving aspirin than in those receiving placebo or ER‐DP. No significant additional bleeding was observed with the aspirin‐plus‐ER‐DP combination compared with aspirin alone. The 3.4% ARR with aspirin plus ER‐DP compared with aspirin alone suggests an NNT of 34 for 2 years to prevent 1 recurrent stroke.63 In addition, the ESPS‐2 data meta‐analysis combined with 14 smaller trials of aspirin and dipyridamole was found to reduce the odds of nonfatal stroke by 23% relative to aspirin monotherapy.64
The European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) was designed to assess the efficacy and safety of aspirin plus dipyridamole versus aspirin alone for secondary prevention of cardiovascular events in patients with ischemic stroke of presumed arterial origin.65 In this trial, 2739 patients were randomly assigned to aspirin (30325 mg/day) with or without dipyridamole (200 mg twice daily) within 6 months of TIA or minor stroke of presumed arterial origin. The primary outcome was a composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. Median aspirin dose was 75 mg/day in both treatment groups, and ER‐DP was used by 83% of the patients in the combination group. The primary outcome occurred in 173 (13%) of patients receiving aspirin plus dipyridamole and in 216 (16%) of those receiving aspirin alone (HR, 0.8; 95% CI, 0.660.98; ARR, 1.0% per year, 95% CI, 0.1%1.8%). The NNT was 33 over 3.5 years to prevent 1 primary outcome with aspirin plus dipyridamole.65 These results, confirming those of ESPS‐2, strongly suggest that use of combination aspirin plus ER‐DP among patients with recent brain ischemia provides significant benefit compared with aspirin alone, without additional adverse effects.
Ticlopidine
Ticlopidine was found to be more effective than aspirin or placebo in risk reduction for recurrent stroke.66 However, the results of several studies showed that its use was associated with serious adverse effects, such as gastrointestinal events, neutropenia, skin rash, and thrombotic thrombocytopenic purpura.66, 67 The more recent African American Antiplatelet Stroke Prevention Study (AAASPS), which included more than 1800 stroke patients, showed that 250 mg of ticlopidine twice daily was no more effective than 325 mg of aspirin twice daily in an African American population.68 Overall, ticlopidine use for prevention of recurrent stroke is not supported by trial data, especially considering the substantial risk of adverse effects.
Anticoagulation
In an additional arm of the ESPRIT trial, 1068 patients were randomly assigned either anticoagulants (target international normalized ratio [INR], 2.03.0) or aspirin (30325 mg/day) within 6 months of a TIA or minor stroke of presumed arterial origin (Table 4).69 In a post hoc analysis, anticoagulants were also compared with the combination of aspirin and dipyridamole (200 mg twice daily). The primary outcome was the composite of death from all vascular causes, nonfatal stroke, nonfatal MI, or major bleeding complication, whichever occurred first. The primary event was observed in 20% of patients (106 of 523) receiving anticoagulants compared with 16% of patients (82 of 509) receiving aspirin plus dipyridamole (HR, 1.31; 95% CI, 0.981.75). The risk for major bleeding was at least 60% lower in patients receiving aspirin plus dipyridamole compared with anticoagulants (2% versus 9%; HR, 4.37; 95% CI, 2.278.43).69 These data confirm that the combination of aspirin plus dipyridamole is more effective than aspirin alone or warfarin for secondary prevention of stroke in patients with stroke of arterial origin.
The Warfarin Aspirin Recurrent Stroke Study (WARSS) compared warfarin (target INR, 1.42.8) versus aspirin (325 mg/day) for the prevention of recurrent ischemic stroke among 2206 patients with a noncardioembolic stroke (Table 4).70 Results of this randomized, double‐blind, multicenter trial showed no significant difference in the rates of recurrent stroke or death (warfarin, 17.8%; aspirin, 16.0%). Warfarin and aspirin were also associated with similar rates of major bleeding (2.2% and 1.5% per year, respectively). Although there were no differences between the 2 treatments, the potential increased risk of bleeding and cost of monitoring were considered in the recommendation of the AHA/ASA to choose antiplatelets over anticoagulants in the setting of noncardioembolic stroke.5
The Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) trial was designed to test the efficacy of warfarin (target INR, 2.03.0 [mean, 2.5]) versus aspirin among patients with >50% angiographically documented intracranial stenosis (Table 4).71 WASID was stopped prematurely because of warfarin's association with significantly higher rates of adverse events and evidence of no benefit over high‐dose aspirin (1300 mg/day). During a mean follow‐up of 1.8 years, adverse events in the 2 groups were death (aspirin, 4.3%, vs. warfarin, 9.7%; HR, 0.46; 95% CI, 0.230.90; P = .02), major hemorrhage (aspirin, 3.2%, vs. warfarin, 8.3%; HR, 0.39; 95% CI, 0.180.84; P = .01), and MI or sudden death (aspirin, 2.9%, vs. warfarin, 7.3%; HR, 0.40; 95% CI, 0.180.91; P = .02). The primary end point (ischemic stroke, brain hemorrhage, and nonstroke vascular death) occurred in approximately 22% of patients in both treatment arms (HR, 1.04; 95% CI, 0.731.48; P = .83).
Statins
Statins reduce the risk of stroke among patients with vascular disease, primarily through LDL cholesterol reduction.72 In the Heart Protection Study (N = 20,536), treatment with simvastatin 40 mg resulted in a 25% relative reduction in the first‐event rate for stroke (P < .0001) and a 28% reduction in presumed ischemic strokes (P < .0001) in patients with cerebrovascular disease, other occlusive vascular disease, or diabetes. No apparent difference in strokes was attributed to hemorrhage (0.5% vs. 0.5%; P = .8). Among patients with preexisting cerebrovascular disease (n = 3280), simvastatin therapy resulted in a 20% reduction in the rate of any major vascular event (P = .001).72
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial examined the effect of high‐dose atorvastatin specifically on secondary prevention of stroke in patients who had a recent history of stroke or TIA and LDL cholesterol levels of 100190 mg/dL (2.64.9 mmol/L) but no known coronary disease.73 In this double‐blind, randomized, placebo‐controlled study, 4731 patients received 80 mg of atorvastatin or placebo. The primary end point was fatal or nonfatal stroke. The mean LDL cholesterol level was 73 mg/dL (1.9 mmol/L) in patients receiving atorvastatin and 129 mg/dL (3.3 mmol/L) in patients receiving placebo. During a median follow‐up of 4.9 years, the incidence of recurrent stroke was lower among patients receiving atorvastatin, with 265 patients (11.2%) experiencing fatal or nonfatal stroke versus 311 (13.1%) of those receiving placebo (5‐year absolute reduction in risk, 2.2%; adjusted HR, 0.84; 95% CI, 0.710.99; P = .03; unadjusted P = .05). Eighty‐seven percent of patients in both treatment groups were receiving concomitant antiplatelet therapy, and 65% were receiving antihypertensives. Atorvastatin treatment resulted in a significant reduction in the risk of fatal stroke but not nonfatal stroke.
In SPARCL, the reduction in risk of fatal or nonfatal stroke, which included hemorrhagic stroke, was maintained despite increased incidence of hemorrhagic stroke with atorvastatin (55 of 273, 20%) versus placebo (33 of 307, 11%).73 The primary end point (fatal and nonfatal strokes) was inclusive of hemorrhagic stroke. Therefore, these results indicate that the benefit seen with atorvastatin therapy was greater than the potential risk of hemorrhagic stroke. High‐dose atorvastatin should be considered for routine secondary prevention on the basis of these findings.
Several studies have evaluated the efficacy of statin therapy in primary prevention of stroke; however, statins were not associated with a decrease in the risk of hemorrhagic stroke.72, 74, 75 Therefore, the potential risk of recurrent hemorrhagic stroke should be considered prior to initiating statin therapy. There is some evidence to suggest that statins can reduce stroke incidence, even in those patients with normal lipid levels, presumably via lowering blood pressure.76
Antihypertensives
High blood pressure is a strong risk factor for initial and recurrent stroke. It is well established that lowering blood pressure reduces the risk of both fatal and nonfatal stroke in a variety of patient groups. The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) quantified the effects of treating hypertension on long‐term disability and dependency among patients with cerebrovascular disease.77 In this randomized, double‐blind, placebo‐controlled study, 6105 patients with a history of stroke or TIA were randomly assigned to receive perindopril 4 mg with or without a diuretic or to receive a placebo. Treatment with perindopril reduced the rate of disability, compared with placebo (19% vs. 22%; adjusted odds ratio, 0.76; 95% CI, 0.650.89; P < .001), primarily by reducing the incidence of recurrent stroke. The NNT for 4 years was 30 (95% CI, 1979) to prevent 1 case of long‐term disability. Interestingly, treatment reduced the risk of stroke in both hypertensive and nonhypertensive patients.78
SUMMARY OF GUIDELINES FOR SECONDARY PREVENTION OF STROKE
The AHA/ASA, American College of Chest Physicians (ACCP), and National Stroke Association (NSA) have developed and published practice guidelines for the management of TIA, with detailed information on secondary prevention of stroke.5, 79, 80 The key recommendations from these 3 organizations are summarized in Table 5 .5, 79, 80 This section summarizes the current guidelines regarding the use of antiplatelets and anticoagulants for the secondary prevention of stroke.
| AHA/ASA5 | NSA79 | ACCP80 | |
|---|---|---|---|
| |||
| Extracranial carotid artery disease | |||
| Hemodynamically significant stenosis 70%, or 50%69% depending on patient‐specific factors | |||
| ○ Carotid endarterectomy* | Class I, level A | Category 1 | No recommendations |
| Nonhemodynamically significant stenosis; stenosis <50% | |||
| ○ Carotid endarterectomy not indicated | Class III, level A | Category 1 | No recommendations |
| Atrial fibrillation | |||
| Long‐term anticoagulation (adjusted‐dose warfarin) | Class I, level A | Category 1 | Grade 1A |
| Aspirin (325 mg/day), if anticoagulants contraindicated | Class I, level A | Category 1 | Grade 1A |
| Mitral valve prolapse | |||
| Long‐term antiplatelet therapy | Class IIa, level C | Category 3 | Grade 1C+ |
| Prosthetic heart valves | |||
| Anticoagulants | Class I, level B | Category 1 | Grade 1C+ |
| Plus antiplatelets (if anticoagulants inadequate) | Class IIa, level B | Category 3 | Grade 1C |
Antiplatelets Versus Anticoagulants
The latest guidelines from the AHA/ASA and the ACCP recommend the use of anticoagulants (adjusted‐dose warfarin) for the secondary prevention of stroke in patients with persistent or paroxysmal atrial fibrillation and in those with artificial heart valves.5, 80 Warfarin therapy (INR, 2.03.0) is also a reasonable option for secondary prevention of stroke in TIA patients with dilated cardiomyopathy. Although warfarin may be prescribed to reduce cardioembolic events in this population, it is controversial whether there is benefit to the use of warfarin in patients with cardiac failure or a reduced left ventricular ejection fraction.81, 82 The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) was initiated to evaluate warfarin versus aspirin 162 mg/day or clopidogrel 75 mg/day in patients with symptomatic heart failure in sinus rhythm with an ejection fraction less than or equal to 35%, but was terminated for poor recruitment.83 Results of observational studies have shown that treatment with warfarin may reduce the risk of recurrent embolism in those with rheumatic mitral valve disease.5, 84
In contrast, for patients with noncardioembolic stroke or TIA, antiplatelet agents are recommended for the secondary prevention of stroke and prevention of other cardiovascular events.5, 79, 80, 85
Currently, there are no data from prospective, randomized, controlled studies to support the use of intravenous heparin or warfarin in patients with carotid or vertebral dissection. The use of anticoagulation in patients with cerebral hemorrhage is influenced by several factors, such as type of hemorrhage, patient age, risk factors for recurrent hemorrhage, and indication for anticoagulation. The risk of recurrent hemorrhage must be weighed against the risk of ischemic cerebrovascular event. The AHA/ASA guidelines recommend that in patients with intracranial hemorrhage, subarachnoid hemorrhage, or subdural hematoma, all anticoagulants and antiplatelets should be discontinued during the acute period of at least 12 weeks posthemorrhage and that the anticoagulant effect should be reversed immediately with appropriate agents.5
FUTURE DEVELOPMENTS
One of the largest stroke prevention trials currently ongoing is the Prevention Regimen for Effectively avoiding Second Strokes (PRoFESS) study. The PRoFESS trial is a large (N = 20,333), randomized, double‐blind, placebo‐controlled, multinational study comparing the efficacy and safety of aspirin plus ER‐DP with that of clopidogrel and the efficacy of telmisartan versus placebo in the presence of background blood pressure treatments in preventing recurrent stroke.86 The primary outcome of the study is time to first recurrent stroke. Recently, the baseline demographics were published.86 The mean age of patients was 66.1 years at enrollment, 36% of patients were women, and mean time from event to randomization was 15 days (40% randomized within 10 days). Most participants had had a stroke of arterial origin (29% large vessel disease and 52% small vessel disease), whereas 2% had had a stroke due to cardioembolism and 18% due to other causes. These baseline data suggest that the trial involves a representative international population of patients with stroke. The PRoFESS trial will provide additional insight into the benefits of the combination of aspirin plus ER‐DP for secondary prevention of stroke in addition to providing direct comparison of efficacy with clopidogrel. The latest information on this and other ongoing stroke prevention trials can be accessed at
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
- ,,American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117:e25–e146.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e140.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Circulation.2006;113:e409–e449.
- .Stroke: early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium.Stroke.1994;25:1877–1881.
- ,,, et al.Deficiency of both protein C and protein S in a family with ischemic strokes in young adults.Neurology.1994;44:1238–1240.
- ,,, et al.Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment.Stroke.1993;24:35–41.
- ,.Cardioembolic stroke.Curr Atheroscler Rep.2006;8:310–316.
- ,.The proximal aorta: a source of stroke.Baillieres Clin Neurol.1995;4:207–220.
- ,,,,,.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,.Evaluation and management of transient ischemic attack: an important component of stroke prevention.Nat Clin Pract Cardiovasc Med.2007;4:310–318.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- Bader MK,Littlejohns LR, eds.AANN Core Curriculum for Neuroscience Nursing.4th ed.Philadelphia, PA:Saunders;2004.
- ,,, et al.Italian guidelines for stroke prevention and management: synthesis and recommendations. Stroke Prevention and Educational Awareness Diffusion.4th ed.Milan, Italy:Hyperphar Group SpA;2005. Available at: http://www.spread.it/SpreadEng/SPREAD_ENG_4thEd.pdf. Accessed January 29, 2008.
- ,,.Atrial fibrillation as an independent risk factor for stroke: the Framingham Study.Stroke.1991:22:983–988.
- ,,, et al.Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke.Stroke.2006;37:2531–2534.
- .Cerebral vasculitis: imaging signs revisited.Neuroradiology.2007;49:471–479.
- ,,, et al.Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning.Radiology.2007;244:532–540.
- ,,.Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review.Stroke.2003;34:1324–1332.
- ,,, et al.Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing.Stroke.2002;33:2003–2008.
- ,,, et al.Neurologic complications of cerebral angiography.AJNR Am J Neuroradiol.1994;15:1401–1407.
- ,,,,,.Frequency of postoperative carotid duplex surveillance and type of closure: results from a randomized trial.J Vasc Surg.2000;32:1043–1051.
- ,,, et al.The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial.Neurology.2007;68:2099–2106.
- ,,,,.Cigarette smoking as a risk factor for stroke: the Framingham Study.JAMA.1988;259:1025–1029.
- ,.Meta‐analysis of relation between cigarette smoking and stroke.BMJ.1989;298:789–794.
- .Moderate alcohol consumption and stroke: the epidemiologic evidence.Stroke.1989;20:1611–1626.
- .Does alcohol prevent or cause stroke?Cerebrovascular Dis.1995;5:379.
- ,,, et al.Moderate alcohol intake, increased levels of high‐density lipoprotein and its subfractions, and decreased risk of myocardial infarction.N Engl J Med.1993;329:1829–1834.
- ,.Alcohol, lipids and lipoproteins. In:Zakhari S,Wassef M, eds.National Institutes of Health: Alcohol and the Cardiovascular System: Research Monograph. NIH publication 96‐4133.Washington, DC:National Institutes of Health;1996;31:369–391.
- ,,,,.Inhibition of platelet aggregation in whole blood by alcohol.Thromb Res.1995;78:107–115.
- ,,.Sustained inhibition of whole‐blood clot procoagulant activity by inhibition of thrombus‐associated factor Xa.Arterioscler Thromb Vasc Biol.1996;16:1285–1291.
- ,.Binge drinking and ambulatory blood pressure.Hypertension.1999;33:79–82.
- ,,, et al.Light‐to‐moderate alcohol consumption and risk of stroke among US male physicians.N Engl J Med.1999;341:1557–1564.
- .The influence of obesity on health (second of two parts).N Engl J Med.1974;291:226–232.
- ,,, et al.Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study.Stroke.2003;34:1586–1592.
- ,,.Physical activity and stroke risk: a meta‐analysis.Stroke.2003;34:2475–2481.
- ,,,,,.Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients: the Heart Outcomes Prevention Evaluation Study Investigators.N Engl J Med.2000;342:145–153.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:776–785.
- ,,, et al. American Heart Association; American Stroke Association Stroke Council.Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report.JAMA.2003;289:2560–2571.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- American Diabetes Association.ADA clinical practice recommendations.Diabetes Care.2004;27:S1–S143.
- ,,,,.Stroke patterns, etiology, and prognosis in patients with diabetes mellitus.Neurology.2004;62:1558–1562.
- ,,, et al.Incidence rates of first‐ever ischemic stroke subtypes among blacks: a population‐based study.Stroke.1999;30:2517–2522.
- ,,, et al.Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US.Diabetes Care.2001;24:1936–1940.
- ,,.Prevention and treatment for development and progression of diabetic macroangiopathy with pioglitazone and metformin [in Japanese].Nippon Rinsho.2006;64:2119–2125.
- American Diabetes Association.Standards of medical care for patients with diabetes mellitus.Diabetes Care.2003;26:S33–S50.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines.Circulation.2004;110:227–239.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high‐risk patients.BMJ.2002;324:71–86.
- Antiplatelet Trialists' Collaboration.Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.BMJ.1994;308:81–106.
- International Stroke Trial Collaborative Group.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic stroke.Lancet.1997;349:1569–1581.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group.N Engl J Med.1991;325:1261–1266.
- ,,, et al.Aspirin resistance in secondary stroke prevention.Acta Neurol Scand.2006;113:31–35.
- CAPRIE Steering Committee.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- ,,,,,.Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus.Am J Cardiol.2002;90:625–628.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.;CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.J Am Coll Cardiol.2007;49:1982–1988.
- ,,, et al.European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- ,,,,.Dipyridamole bioavailability in subjects with reduced gastric acidityJ Clin Pharmacol.2005;45:845–850.
- Thrombosis Interest Group of Canada. Practice guidelines [on‐line monograph]. Updated yearly. Available at: http://www.tigc.org/eguidelines/strokeprevention.htm. Accessed May 16, 2001.
- ,.Dipyridamole plus aspirin in cerebrovascular disease.Arch Neurol.1999;566:1087–1092.
- ESPRIT Study Group.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high‐risk patients. Ticlopidine Aspirin Stroke Study Group.N Engl J Med.1989;321:501–507.
- ,,,,,.Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases.Ann Intern Med.1998;128:541–544.
- ,,, et al.;African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial.JAMA.2003;289:2947–2957.
- ;ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- ,,, et al. for the Warfarin‐Aspirin Recurrent Stroke Study Group.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345:1444–1451.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- ,,, et al. Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- ,,, et al.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355:549–559.
- ,,, et al.Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): a randomised controlled trial.Lancet.2002;360:1623–1630.
- Heart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial.Lancet.2002;360:7–22.
- ,,,.Analysis of antihypertensive effects of statins.Curr Hypertens Rep.2007;9:175–183.
- Perindopril Protection Against Recurrent Stroke Study PROGRESS Collaborative Group.Effects of a perindopril‐based blood pressure‐lowering regimen on disability and dependency in 6105 patients with cerebrovascular disease.Stroke.2003;34:2333–2338.
- PROGRESS Collaborative Group.Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- .A plea for a clinical trial of anticoagulation in dilated cardiomyopathy.Am J Cardiol.1990;65:914–915.
- .Antithrombotics for left‐ventricular impairment?Lancet.1998;351:1904.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infarction.N Engl J Med.1997;336:251–257.
- ,,,,.Usefulness of anticoagulant therapy in the prevention of embolic complications of atrial fibrillation.Am Heart J.1986;112:1039–1043.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38:1655–1711.
- ,,;Steering Committee; PRoFESS Study Group.Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus asa with clopidogrel) and telmisartan versus placebo in patients with strokes. The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS).Cerebrovasc Dis.2007;23:368–380.
Copyright © 2008 Society of Hospital Medicine
Systems Approach to Stroke Care
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16
Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16

Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
Despite the considerable national attention drawn to the need for improved secondary stroke prevention, a gap remains between evidence and application for stroke and other vascular events. Experience with the Coverdell stroke registry has shown that a minority of acute stroke patients receive the care recommended in established guidelines.1 Data collected from 4 registry centers in the United States showed a consistent lack of appropriate diagnostics, patient education, and initiation of drug therapies proven to reduce the risk of recurrent stroke.1
According to a report from the Committee on the Quality of Healthcare in America published in 2001, suboptimal treatment as well as inefficient use of health resources can be largely attributed to fragmentation of health care delivery in the management of various diseases in the United States.2 In response to these findings, the American Stroke Association (ASA) has established recommendations for the development of stroke systems of care. The objective of a systems approach is to integrate preventive and treatment services and provide patients with evidence‐based care.3
During hospitalization for acute stroke, immediate treatment must focus on minimizing stroke progression, avoiding common complications, and preventing recurrent stroke. Prior to discharge, patients need to be educated about the importance of lifestyle modifications and pharmacotherapies to reduce their risk of a recurrence of the stroke and other atherosclerotic vascular events.3 As the physicians who focus on inpatient care, hospitalists are likely to be responsible for participating in and coordinating the multidisciplinary team that provides treatment and services to stroke patients. Hospitalists also must facilitate the transition from inpatient to outpatient care. Hospitalists are in a position to help educate stroke patients about prevention strategies throughout the hospitalization period. These functions provide hospitalists with the opportunity to lead, coordinate, and participate in stroke systems care at their institutions.
The present article discusses the components of stroke systems care recommended by the ASA and the best‐practices recommendations from the recent hospitalist roundtable discussion on routine acute stroke care. The national treatment guidelines and clinical trials supporting the recommendations of the hospitalist roundtable participants have been discussed in the article in this supplement by Dr. Likosky et al, as well as in the patient scenarios article in this supplement by Dr. Lee et al. Some of the anticipated barriers and pitfalls that may be encountered, along with potential solutions, are also discussed. Hospitalists may be able to use this review to adapt feasible components of the systems care for stroke management to improve care at their institutions.
WHAT IS STROKE SYSTEMS CARE?
A stroke system is coordinated stroke care along the entire continuum from primary prevention to rehabilitation. Postemergency department inpatient care for patients with acute stroke, also referred to as subacute care, is only one component of the community‐based stroke systems of care recommended by the ASA (Fig. 1).3 In this model, regional stroke systems identify hospitals that are acute stroke capable and determine that those institutions use clinical pathways that reflect well‐established standards of care and nationally recognized guidelines.3 In this broad sense of the term, stroke systems function to organize and coordinate the various agencies and health care providers responsible for caring for patients with stroke, from the first call to emergency services through postdischarge medical care and rehabilitation (Table 1). The subacute phase of care provides the bridge from management of the medical emergency to discharge and is central to secondary stroke prevention.

|
| 1. Ensure effective interaction and collaboration among agencies, services, and people involved in providing prevention and timely identification, transport, treatment, and rehabilitation of individual stroke patients in a locality or region. |
| 2. Promote the use of an organized standardized approach at each facility and component of the system. |
| 3. Identify performance measures (both process and outcomes measures) and include a mechanism for evaluating the effectiveness through which the entire system and its individual components continue to evolve and improve. |
RATIONALE FOR HOSPITAL‐BASED STROKE SYSTEMS
The Preventing Recurrence of Thrombo‐embolic Events through Coordinated Treatment (PROTECT) program provides proof of concept.4 The PROTECT program was implemented at a large teaching hospital to improve diagnosis, treatment, and secondary prevention for patients with ischemic stroke.4 Four medication goals and instruction in 4 lifestyle interventions were chosen as indicators of program impact. In the first year after PROTECT was started, 100% of eligible patients received instruction in all 4 areas of lifestyle change prior to discharge.4 In the year following implementation of PROTECT, the rate of appropriate prescribing of antithrombotics was 98%. Appropriate prescribing of angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, and thiazide diuretics was significantly increased from pre‐PROTECT levels.4 After 3 months of follow‐up, patient adherence to therapy remained high.5 The final results of the PROTECT program are not yet available; however, it is reasonable to expect that increased use of evidence‐based therapy and good patient adherence to these proven therapies will have led to better patient outcomes, including lower rates of recurrent stroke.
Patient outcomes data are available for a related initiative for treatment of patients hospitalized with myocardial infarction. Compared with the year prior to implementation of the Cardiac Hospitalization Atherosclerotic Management Program (CHAMP), more patients who were involved in the CHAMP intervention achieved low‐density lipoprotein cholesterol levels P < .001). In addition, these patients achieved a 57% reduction in recurrent myocardial infarction.6
These 2 studies indicate a benefit of establishing hospital‐based stroke systems; however, these studies are the initial steps, and each has limitations. For example, neither study was a prospective, randomized trial with a concurrent control group.4, 6 In addition, PROTECT data were not evaluated by independent audit but by individuals who were aware of the program goals, and limited data were available regarding contraindications to therapy.4 CHAMP did not assess adherence to nonpharmacologic interventions or the effect of surgical interventions.6 Large, randomized, controlled trials are needed to better understand the impact of such systems. Although larger evidence‐based trials are needed, it is important to review available information on stroke systems to adapt those components that align with each institution's available resources.
ESTABLISHING HOSPITAL‐BASED STROKE SYSTEMS
Several barriers exist to establishing a stroke systems care program, as detailed in Table 2. The support and involvement of the hospital administration is essential to success, as is multidisciplinary agreement that such a program will benefit patients.
| Barriers | Solutions |
|---|---|
| |
| 1. Lack of proof of concept. | 1. PROTECT demonstrates improved stroke care. |
| 2. Lack of ownership: acute versus chronic disease dilemma. | 2. View hospital as capture point for patients with chronic diseases. |
| 3. Lack of financial incentives. | 3. JCAHO/NCQA will measure and report to payers. |
| 4. Communication gapsneurologists, hospitalists, and primary care physicians. | 4. Education and mobilization of case management teams. |
| 5. Poor standardization of orders and testing procedures. | 5. Written protocols for diagnosis and treatment; written orders. |
| 6. Lack of tools and resources. | 6. JCAHO, Get with the Guidelines, and PROTECT Web sites. |
Other potential points of resistance revolve around the financial impact of implementing a stroke systems approach to care. The proposed stroke systems care plan is consistent with meeting nationally recognized quality improvement standards; however, the current health care market forces demand accountability for health care expenditures. Increasingly, payers are turning to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) evaluations to determine quality of care at various institutions. These programs encourage the use of standardized treatment protocols consistent with the concept of systems approach to care. Moreover, stroke care is a JCAHO quality measure and thus may have a financial impact on hospitals. It is possible that implementing standardized procedures for stroke care may reduce the cost of care. Information about the JCAHO Disease Specific Certification for Acute Stroke Care can be accessed at
Once there is agreement that a stroke system should be developed, a multidisciplinary team should be established. A multidisciplinary team may include hospitalists, neurologists, neurosurgeons, emergency medicine physicians, diagnostic and interventional radiologists, nurses, physiotherapists, occupational therapists, speech and language therapists, and social workers. However, the components of the multidisciplinary team may vary depending on the available staff and financial resources at different stroke centers. Assuring all participants in the system that their input is valued can improve communication among stroke specialists, hospitalists, and primary care clinicians. This team is responsible for evaluation of current procedures and development of algorithms, discharge forms, patient education, and preprinted orders.
The task of developing a cohesive plan for stroke care may appear onerous. Existing diagnostic and treatment procedures may be poorly designed or organized. However, multiple online sources provide tools for every aspect of stroke systems care. Information about evidence‐based stroke care practices is available as part of the American Heart Association (AHA)/ASA Get with the GuidelinesStroke program and can be accessed at
A stroke system of care is a dynamic process. The multidisciplinary team may also be responsible for continuous monitoring and reporting of the efficiency and impact of the system and providing feedback to other staff and administration. Protocols should be revised regularly to account for new evidence‐based treatments and to streamline their use. The Canadian Stroke Systems Coalition recommends that a comprehensive and efficient system include prevention, prehospital and emergency care, hospital care, rehabilitation, reintegration into the community, surveillance, and research.11 Hospital staff should be educated in core competencies in hospital medicine as well as any changes to protocols made over time. Protocols that facilitate communication among health care providers should also be developed, and hospitalists may play a central role in this process. Accurate and timely transfer of patient information from the emergency department to the stroke center or ward is imperative.
FOCUSING ON INPATIENT CARE
Clinical pathways for inpatient care should be designed to limit stroke progression as much as possible.3 The Brain Attack Coalition (BAC) provides a resource for clinical pathways implemented at various institutes in the United States, including the Stanford Stroke Center, the Cleveland Clinic Foundation, and Thomas Jefferson University Hospital, among others (
A neurologist should be available to the stroke system patients at all times, and ideally, all acute stroke patients should be evaluated by a neurologist specializing in the evaluation and treatment of patients with stroke.14 There are several stroke scales available to evaluate stroke patients, including the Barthel Index, the Glasgow outcome scale, the Modified Rankin Scale, the National Institutes of Health Stroke Scale, and the Hunt and Hess Classification of Subarachnoid Hemorrhage (
Common complications of stroke, such as myocardial infarction, deep vein thrombosis, pulmonary embolism, urinary tract infections, aspiration pneumonia, dehydration, poor nutrition, skin breakdown, and metabolic disorders, should be anticipated, and preventive steps should be taken. The measures to prevent the above complications of stroke need to be initiated in the emergency department.3
Management of existing comorbid conditions is another key part of subacute stroke care. Given that 85% of all hospitalists have a background in internal medicine, management of comorbid conditions such as diabetes and hypertension is an area in which hospitalists have professional competence. Patient history and use of prescription medications prior to stroke should be reviewed whenever possible and incorporated into short‐term and long‐term treatment plans. Patients with diabetes in particular may benefit more from rigorous control of blood pressure and lipids compared with other patients.16
Secondary stroke prevention should start as early as considered safe. Diagnosis of stroke subtype, often accomplished in the emergency department, establishes suitability for antithrombotics and optimal management strategy. Patients who receive a diagnosis of stroke secondary to cardioembolic atrial fibrillation should be treated with an anticoagulant after the acute period. Aspirin can be used for those individuals unable to use anticoagulants.16 For those individuals with stroke of noncardioembolic origin, particularly those with atherosclerosis and lacunar or cryptogenic infarcts, antiplatelet agents are recommended.14
A multimodal prevention strategy is recommended to manage blood pressure and dyslipidemia poststroke. An algorithm for managing blood pressure soon after stroke has been developed by the PROTECT program (Fig. 2).10 Antihypertensives, usually a combination of an ACE inhibitor and a thiazide diuretic, can be initiated at low doses 48‐72 hours after stroke. A longer delay is recommended for patients with large infarcts or evidence of uncontrolled hypertension. ARBs may be substituted for ACE inhibitors.10 Target blood pressures should be determined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.17 In general, even a reduction of 10/5 mm Hg has been shown to be beneficial.16

Statins are recommended for all patients with elevated serum lipids unless treatment with statins is contraindicated. The recommended target level for low‐density lipoprotein cholesterol is below 100 mg/dL for individuals with coronary heart disease and symptomatic atherosclerosis. A target below 70 mg/dL may be appropriate for patients at very high risk.16
Prior to discharge, patients or their caregivers should be given prescriptions adequate to cover the time until postdischarge follow‐up visit. The responsible persons need to be made aware that some medications such as antihypertensives will require dosage adjustments by an outpatient physician, and the timing of the follow‐up visit may need to be arranged accordingly.
The importance of stroke risk reduction should be part of predischarge patient education, along with a list of the warning signs of stroke. Adherence to the treatment regimen, including lifestyle changes and medications, should be emphasized. Patients or their caregivers should be educated about identifying adverse events and a plan to address them. Understanding that some adverse effects (eg, headache with aspirin plus extended‐release dipyridamole) are likely to be transient may prevent unnecessary discontinuation of treatment and reduce anxiety.
Patient and caregiver education can be reinforced by providing standardized patient education materials that can be found in the Stroke Resource Room at the Society of Hospital Medicine Web site (
Transfer of patient information to outpatient health care providers is a critical step in stroke systems care. Notes indicating any need for medication dose adjustment must be included. Discharge summaries should be available to primary care providers, neurologists, and rehabilitation specialists prior to follow‐up visits. The use of electronic forms that can be faxed or sent by E‐mail can shorten delivery time considerably. In lieu of electronic delivery, physician letters can be used, and prototypes are available at the resource Web sites. Whenever possible, a follow‐up phone call to the primary care physician provides the best means to ensure clear communication.
SUMMARY
Hospitalists are well qualified to lead quality focused patient care initiatives at their institutions. Use of standardized protocols to reduce the risk of secondary stroke is proven to increase appropriate prescribing at discharge, which in turn improves patient adherence to evidence‐based therapy. Multidisciplinary communication, including communication with outpatient clinicians, facilitates the transition from inpatient to outpatient health care providers.
In addition to improving patient care, use of standardized protocols is tracked by JCAHO and offers assurance to payers that a particular hospital and its staff are committed to quality care. Establishing protocols is made relatively easy by the online availability of materials that can be adapted to various hospital settings.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
- , for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- Committee on Quality of Health Care in America, Institute of Medicine.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academies Press;2001.
- ,,, et al.American Stroke Association's Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association's Task Force on the Development of Stroke Systems.Circulation.2005;111:1078–1091.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP).Am J Cardiol.2001;87:819–822.
- Joint Commission on Accreditation of Hospital Organizations web site. Available from URL: http://www. jointcommission.org/. Accessed September 12, 2007.
- American Stroke Association. Get with the Guidelines. Available at: www.strokeassociation.org/presenter.jhtml? identifier = 1200037. Accessed September 12, 2007.
- Society for Hospital Medicine. Stroke Research Room. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Quality_Improvement_Resource_Rooms164:1853–1855.
- Brain Attack Coalition. Pathways. Available at: http://stroke‐site.org/pathways/pathways.html. Accessed January 28, 2008.
- ,,,,,.Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I.Stroke.1999;30:2631–2636.
- ,,, et al.Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition.Stroke.2005;36:1597–1618.
- Brain Attack Coalition. Stroke scales. Available at: http://www.stroke‐site.org/stroke_scales/stroke_scales.html. Accessed January 28, 2008.
- ,,, et al.American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Stroke.2006;37:577–617.
- ,,, et al.Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.JAMA.2003;42:1206–1252.
- American Heart Association. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200000. Accessed September 12, 2007.
Copyright © 2008 Society of Hospital Medicine
Challenging Patient Cases
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
The risk of recurrent stroke is high following an ischemic stroke or transient ischemic attack (TIA).16 Within the first 90 days following an initial TIA, between 4.8% and 18.3% of individuals will have an ischemic stroke, with many experiencing an ischemic event within the first 27 days.14 The risk of subsequent stroke in a stroke survivor is high as well4.2% at 6 months, 6.5% at 1 year, and 11.8% at 3 years.5 The management of these patients poses substantial challenges for the health care professional. Prevention of secondary stroke, with its risk for greater morbidity and mortality, is a priority. However, depending on the cause of the event, patient comorbidities, and other factors, the most effective therapeutic strategies may differ. For example, cardioembolic strokes, which constitute approximately 20% of ischemic strokes, are treated with anticoagulants, whereas strokes of noncardioembolic origin are usually treated with antiplatelet agents.7, 8 Other risk factors or variables such as recent stent placement or reduced left ventricular ejection fraction (LVEF) may affect therapeutic decisions as well, although in many cases clear data are not available to direct these difficult decisions. Thus, although antiplatelet agents, including aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole, prevent strokes, the choice of agent depends on the individual patient risk profile. A number of challenging patient scenarios are explored in this article with the goal of providing a context for some of the more recent trial data.
RECENT STENT PLACEMENT
In 2004, there were approximately 663,000 percutaneous coronary interventions (PCIs).9 Stenting after PCI is a common procedure and is used in more than 70% of coronary angioplasty procedures. The addition of stenting to the PCI procedure has improved the outcome for patients, reducing the need for revascularization.10 Because restenosis of the area following stent placement is common, drug‐eluting stents are also used to allow slow release of antiproliferative agents such as sirolimus or paclitaxel.11, 12
Studies such as Percutaneous Coronary InterventionClopidogrel in Unstable Angina to Prevent Recurrent Events (PCI‐CURE) and Clopidogrel for Reduction of Events During Observation (CREDO) have supported the use of up to 8 months of clopidogrel plus aspirin following coronary interventions.13, 14 The European Society of Cardiology PCI guidelines state that in regard to PCI procedures, clopidogrel is superior to aspirin. The guidelines recommend 34 weeks of clopidogrel following stenting in patients with stable angina but up to 12 months in patients receiving brachytherapy. Among patients who have received drug‐eluting stents, clopidogrel therapy should be continued for 612 months. In contrast, aspirin therapy (75100 mg/day) should be continued for life in all these patients.10 In patients who have had a nonST segment elevation myocardial infarction (MI) or who have unstable angina, these guidelines recommend the continuation of clopidogrel (75 mg/day) plus aspirin (100 mg/day) for 912 months after a PCI procedure.10
However, although clopidogrel plus aspirin reduces the incidence of major ischemic events in the period immediately following a stenting procedure, some have suggested that long‐term use of clopidogrel is not supported by the evidence.14 It has been proposed that the sustained beneficial effect of clopidogrel given in the immediate postoperative period may account for much of the long‐term benefit, as has been shown to be true of the glycoprotein IIb/IIIa antagonists.14 However, others caution that in the case of drug‐eluting stents, inhibition of endothelialization of the stent struts by the embedded agents makes these stents more susceptible to thrombosis formation, particularly if therapy with clopidogrel plus aspirin is interrupted.12 It is believed that late stent thrombosis, which has a high mortality rate, is more common with drug‐eluting stents than with bare‐metal stents.12, 15 As a result, many cardiologists recommend at least 12 months of dual antiplatelet therapy with aspirin plus clopidogrel for patients who have received drug‐eluting stents.12 However, given the results of the recent Management of Atherothrombosis in High‐risk Patients with Recent Transient Ischemic Attack or Ischemic Stroke (MATCH) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trials,16, 17 in particular, the high incidence of bleeding events in the clopidogrel plus aspirin group, there are concerns about longer‐term or lifelong therapy with this combination in a population at risk for recurrent stroke.
What about the patient who has undergone a coronary stent placement in the past 12 months and experiences a subsequent ischemic stroke or TIA? The patient should be continued on clopidogrel plus aspirin for the recommended time, as premature discontinuation of antiplatelet therapy increases the risk of stent thrombosis.18 No data are currently available to support decision making regarding these patients. However, it has been suggested that among patients given drug‐eluting stents, extended use of clopidogrel at 6, 12, and 24 months is associated with reduced risk of death or death/MI.18
LOW EJECTION FRACTION
Patients who have had a stroke or TIA and have underlying left ventricular dysfunction are at increased risk of a cardioembolic stroke.8 The reduction in stroke volume creates a condition of stasis in the ventricle that increases the likelihood of coagulation and thromboembolic events.8, 19 Evidence indicates that the risk of stroke is inversely correlated with LVEF; LVEF of 29%35% carries a cumulative 5‐year stroke risk of 7.8%, and LVEF of 28% or below carries a 5‐year risk of 8.9%.8, 20, 21 Data from the Survival and Ventricular Enlargement (SAVE) study showed an 18% increase in the risk of stroke for every 5% decline in LVEF,19, 21 and the Studies of Left Ventricular Dysfunction (SOLVD) trial found a 58% increase in thromboembolic events for every 10% decrease in LVEF among women (P = .01).19, 22 Among patients with low LVEF who have had a stroke, the 5‐year recurrent stroke rate may be as high as 45%.19, 23
Although it would appear that stroke associated with left ventricular dysfunction and a low LVEF may potentially be cardioembolic in origin, risk reduction for recurrent stroke has not been adequately investigated as a primary end point in clinical trials, particularly in the absence of atrial fibrillation.24 Thus, the question of whether antiplatelet or anticoagulant therapy would be more effective has not yet been answered. However, results of secondary end point analyses in the SOLVD and SAVE trials suggested that patients had a lower risk of sudden death, thromboembolism, and stroke with antiplatelet therapy.21, 2426 In an observational analysis of prospectively collected data on patients enrolled in the SAVE trial, use of aspirin reduced the overall risk of stroke by 66% in patients with an LVEF below 28%.21 Warfarin is the standard of care for stroke prevention in atrial fibrillation, and the 2 conditions often coexist. In those patients, warfarin is the recommended therapy.24
In patients with sinus rhythm and a low LVEF, the choice is less clear. The results of the Warfarin/Aspirin Study in Heart failure (WASH) failed to establish efficacy or safety for aspirin in preventing all‐cause mortality, nonfatal MI, and nonfatal stroke in patients with heart failure. Patients treated with aspirin were significantly more likely to be hospitalized for cardiovascular events, especially worsening heart failure.27 The trial found no significant difference for the composite end point between the 3 treatment groups: aspirin, warfarin, or no antithrombotic treatment. However, this was a small trial, and the findings were far from definitive, as the study was designed primarily to be a feasibility study to aid in the design of a larger outcomes study.24 Because of the inconsistent results and lack of well‐designed studies regarding the benefit of aspirin or anticoagulation for secondary stroke prevention in patients with LVEF in the absence of atrial fibrillation, further study is needed.
More recently, results were presented from the Warfarin and Antiplatelet Therapy in Heart Failure Trial (WATCH), which randomized patients with heart failure, sinus rhythm, and LVEF of 35% or below to either aspirin 162 mg, warfarin (target international normalized ratio [INR] 2.53.0), or clopidogrel.28, 29 Two major comparisons were plannedwarfarin versus aspirin and aspirin versus clopidogrel.28 Whereas warfarin therapy was open‐label because of the need to check blood levels, antiplatelet therapy was given in a double‐blind manner. After a mean follow‐up of 23 months, no significant differences were found for the primary composite end point of all‐cause mortality, nonfatal MI, and nonfatal stroke, which occurred in 20.5% of those on aspirin, 19.8% on warfarin, and 21.8% on clopidogrel. However, for the secondary end point of stroke, there was a strong trend favoring warfarin over aspirin: stroke occurred in 0.7% of patients taking warfarin versus 2.1% of those taking aspirin (P = .06).24, 29 However, the WATCH investigators concluded that the question of warfarin's value for patients with low LVEF and sinus rhythm remained unresolved.29
In the absence of clear data, the American Heart Association (AHA)/American Stroke Association (ASA) guidelines on stroke prevention in this patient population recommend either warfarin (INR 2.03.0) or antiplatelet therapy, including aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole (200 mg twice daily), or clopidogrel (75 mg/day).8 Patients with coexisting atrial fibrillation should be treated with warfarin, or if unable to tolerate that agent, aspirin 325 mg/day.8
The Warfarin Versus Aspirin for Reduced Cardiac Ejection Fraction (WARCEF) trial may provide more definitive answers on the best approach for reducing the risk of recurrent stroke in patients with low LVEF. The study will compare warfarin (INR 2.53.0) and aspirin (325 mg/day) in the prevention of all‐cause mortality and all strokes (ischemic and hemorrhagic) in patients with an LVEF of 35% or below but no atrial fibrillation.30 The study has a target enrollment of 2860 patients, who are being recruited at 70 North American and 70 European sites, and it will include patients with recent stroke or TIA.28 The results are anxiously anticipated.
INTRACRANIAL STENOSIS
Stroke patients with symptomatic intracranial atherosclerosis have a high risk of recurrent strokein the range of 10% per yearand this accounts for approximately 8% of ischemic strokes.8, 31, 32 Intracranial stenosis appears to be more common in African Americans and Hispanics than in white patients.31
Recurrent stroke prevention in patients with intracranial stenosis was explored in the Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) study, a multicenter, double‐blind trial. Patients with angiographically verified 50%99% stenosis of a major intracranial artery who had experienced either a stroke or TIA were randomized to either warfarin (target INR 2.03.0) or high‐dose aspirin (1300 mg/day). The primary end point was ischemic stroke, brain hemorrhage, or death from vascular causes other than stroke.33 Mean follow‐up was 1.8 years, and enrollment was stopped after 569 patients had been randomized because of concerns about the safety of warfarin in this patient population.33 The primary end point occurred in 22.1% of those treated with aspirin and 21.8% of those treated with warfarin.33 There were no significant differences between the 2 treatment groups for any of the prespecified secondary end points, including ischemic stroke in any vascular territory and ischemic stroke in the territory of the stenotic intracranial artery.33
The rate of death was significantly higher in the warfarin group (9.7%) than in the aspirin group (4.3%; P = .02). Patients in the warfarin group had higher rates of death from both vascular and nonvascular causes.33 Major hemorrhage was significantly more common in the warfarin group (8.3%) than in the aspirin group (3.2%; P = .01). The investigators concluded that warfarin should not be used as first‐line prevention of recurrent stroke in patients with intracranial stenosis. However, there was a significant association between an INR less than 2 and increased risk of ischemic stroke and major cardiac events (P < .001) as well as a significant increase in major hemorrhages in patients with INRs greater than 3 (P < .001).33
The failure of many patients in the study to remain within the therapeutic INR casts doubt on these results to some extent, although this may actually mirror a common real‐world scenario. Patients were within the therapeutic INR goal only 63% of the time. Furthermore, a nonstandard high dose of aspirin (1300 mg/day) was used, which also may have affected the results.34 Others looking at this data have suggested that aspirin remains an imperfect therapy, with an unacceptably high risk of ischemic stroke and other vascular events, and that anticoagulation may play a role in the period immediately following ischemic stroke or TIA with transition to antiplatelet therapy.34 This would require additional investigation.34
The current AHA/ASA guidelines recommend that for patients with noncardioembolic ischemic stroke or TIA, antiplatelet agents rather than oral anticoagulants be used to reduce the risk of recurrent stroke (class I, level A). Aspirin (50325 mg/day), the combination of aspirin and extended‐release dipyridamole, and clopidogrel are all acceptable options for initial therapy (class IIa, level A).8 The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone (class IIa, level A), and clopidogrel may be considered instead of aspirin alone (class IIb, level B).8 However, data are insufficient at this point to make evidence‐based recommendations between antiplatelet options other than aspirin.8 In patients with significant intracranial stenosis whose symptoms persist despite medical therapy, including antithrombotics, statins, and antihypertensives, endovascular therapy with angioplasty and/or stent placement is an option, but it remains investigational and its value is uncertain.8
CAROTID STENOSIS
Asymptomatic carotid stenosis greater than 50% has been found in 7% of men and 5% of women older than 65 years.35, 36 Among those with asymptomatic carotid stenosis greater than 50%, there is an annual risk of stroke of up to 3.4%.35 In such patients, the benefit of carotid endarterectomy (CEA) is highly dependent on the surgical risk, and if complication rates exceed 3.0%, benefit is eliminated.35 The AHA/ASA guidelines recommend that patients be given treatment for all identifiable risk factors, including statins for dyslipidemia, antihypertensives for hypertension, and aspirin as an antiplatelet agent. In select patients with high‐grade asymptomatic carotid stenosis, CEA performed by a surgeon with a morbidity/mortality rate below 3% is recommended.35 In asymptomatic patients with greater than 70% carotid stenosis, CEA can be an effective therapy. Trial data indicate that the overall 5‐year risk of any stroke or perioperative death is 11.8% for deferred surgery versus 6.4% for immediate endarterectomy (P < .0001).35, 37 Unfortunately, data on the value of stents or angioplasty compared with CEA in this patient population are limited.35
In patients who have had a recent TIA or stroke, carotid stenosis would be considered symptomatic. In these patients, the benefit of CEA is strongly associated with the degree of stenosis. Data from the Carotid Endarterectomy Trialists' Collaboration and North American Symptomatic Carotid Endarterectomy Trial (NASCET) have shown that in patients with stenosis greater than 70%, CEA reduces the absolute 5‐year risk of ischemic stroke by 16.0% (P < .001), whereas in patients with 50%69% stenosis, the 5‐year absolute risk reduction is 4.6% (P = .04). In those with stenosis of 30%49%, there is no effect, and CEA in patients with less than 30% stenosis increases the risk of stroke.38, 39 In patients with 50%69% stenosis, benefit is achieved only if patients at highest risk are selected.40 Recent data have also questioned the typical 4‐ to 6‐week delay before performing a CEA following a nondisabling stroke. Rothwell et al. found that surgery performed within 2 weeks of such a stroke was not associated with increased operative risk.41 Moreover, benefit from CEA fell rapidly within the first few weeks after a TIA or stroke, particularly in women, perhaps reflecting the high risk of recurrent stroke in the period immediately following an initial event.41
Angioplasty or stents have been investigated as alternatives to CEA, but the evidence to date has been disappointing. The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) demonstrated preventive efficacy and major risks similar to those found for CEA after 3 years of follow‐up in 504 patients with carotid stenosis.42 However, a more recent study was stopped prematurely after 527 patients had been enrolled because of a higher incidence of disabling stroke or death at 30 days in the stenting cohort (3.4%) compared with the CEA cohort (1.5%). The 30‐day incidence of any stroke or death was 3.9% after CEA and 9.6% after stenting, yielding a relative risk of 2.5 for stenting.43 The Stent‐Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial has also failed to find benefit for carotid stenting and/or angioplasty in comparison with CEA.44
The AHA/ASA guidelines recommend CEA in patients with ipsilateral severe (70%99%) stenosis and a recent TIA or ischemic stroke (within 6 months). Surgery should be performed by a surgeon with a perioperative morbidity/mortality rate less than 6%.8 In patients with 50%69% stenosis, the advisability of CEA depends on patient factors such as age, sex, comorbidities, and severity of symptoms. Surgery should be performed within 2 weeks of an ischemic event. In patients with severe stenosis in whom CEA would be difficult to perform, carotid angioplasty or stenting may be recommended if performed by practitioners with a morbidity/mortality rate less than 4%6%.8 The Seventh ACCP Conference also recommends that patients undergoing CEA receive aspirin 81325 mg/day prior to and following the procedure.7
ATHEROSCLEROSIS OF THE AORTIC ARCH
Atherosclerosis of the aortic arch contributes significantly as an independent factor to risk of embolic stroke.7 Such plaques can be detected using transesophageal echocardiography; those that are thicker than 45 mm, exhibit ulceration, or have mobile components place individuals at higher risk for stroke.7, 45 The stroke risk associated with aortic arch plaques greater than 5 mm is as high as 33% per year.7, 46
However, data from large‐scale randomized clinical trials on the efficacy of therapeutic interventions in this condition are lacking. Two small trials found efficacy for warfarin in patients with mobile thrombi in the thoracic aorta. In one, patients given oral anticoagulants had better outcomes than those treated with antiplatelet agents, and in the other, warfarin proved to be more effective than no treatment.47, 48 A retrospective trial that looked at 519 patients treated with warfarin, antiplatelet agents, or statins found there was a protective effect of statins, with an absolute risk reduction in embolic events, including ischemic stroke, TIA, and peripheral embolization of 17%, and a relative risk reduction in embolic events of 59%. The odds ratio for embolic events was 0.39 for statins, 0.77 for antiplatelet agents, and 1.18 for warfarin.49 The French Study of Aortic Plaque in Stroke found no significant difference in risk of events between those treated with warfarin and those treated with aspirin; however, this study was not designed as a therapeutic trial, and few patients received warfarin, casting doubt on this finding.45
Given the paucity of data, suggestions for treatment of patients with an aortic arch atheromata are difficult. Certainly, statin therapy, which would address general atherosclerotic risk reduction, can be initiated. Warfarin appeared to be more effective than antiplatelet agents in several of the studies; however some have expressed concern about the possibility of anticoagulation increasing the risk of cholesterol embolism in these patients.7
SYMPTOMATIC CORONARY ARTERY DISEASE
For patients with a history of ischemic stroke or TIA who have symptomatic CAD, their condition must be managed for both stroke and CAD risks. In patients with stable or unstable angina and a history of stroke or TIA, similar risks must be managed. The acute treatment of ACS or symptomatic CAD cannot be adequately addressed here; however, it may involve a number of therapeutic modalities, including PCI, ‐blocker therapy, glycoprotein IIb/IIIa inhibitors, anticoagulant therapy, angiotensin‐converting enzyme (ACE) inhibitors, and clopidogrel plus aspirin, depending on the exact nature of the syndrome.5054 The long‐term management and, in particular, prevention of recurrent stroke in the setting of symptomatic CAD are the focus here. As with a patient with a history of CAD and a recent TIA or stroke (as discussed earlier), patients with symptomatic CAD and TIA or stroke must be managed for multiple risk factors. NCEP guidelines recommend aggressive cholesterol lowering with statin therapy. Hypertension must be addressed as well, and long‐term therapy with ‐blockers and ACE inhibitors has been shown to reduce mortality in patients with ACS and is recommended by the AHA/ASA.5355
Once the acute ACS period has resolved, it is reasonable to address the question of the best possible antiplatelet therapy for long‐term stroke prevention. Long‐term use of clopidogrel plus aspirin is not advisable given the increased risk of bleeding events noted in the MATCH and CHARISMA trials.16, 17 At this point, it would be reasonable to start the patient on aspirin 75150 mg/day, which reduces risk of stroke up to 25%,56, 57 aspirin plus extended‐release dipyridamole, which reduces risk by about 37%,57, 58 or clopidogrel 75 mg/day, which reduces the relative risk for stroke alone by 7.3% compared with aspirin.59 In patients who cannot tolerate or are allergic to aspirin, clopidogrel is a reasonable choice.8
ANTIPLATELET FAILURE
Patients who have failed antiplatelet therapythat is, have gone on to have a recurrent strokeare particularly difficult. It is important to remember that any therapeutic intervention only reduces stroke risk; it does not eliminate it. Keeping that in mind, it is essential to reevaluate and reconsider both the original diagnosis and the etiology of the stroke or TIA. A number of diagnostic alternatives should be considered, including sensory seizure and migraine equivalents, as well as other etiologies, such as atrial fibrillation or cerebral amyloid angiopathy. Therapy may have to be adjusted accordingly, but the patient remains at increased risk for stroke recurrence, and thus preventive therapy is critical.
Several key points should be remembered. As outlined previously in this article, if the stroke is still thought to be noncardioembolic in origin, a reduction in the risk of stroke has not been found for those patients receiving warfarin, an increased dose of aspirin, a combination of antiplatelet agents and warfarin, or clopidogrel plus aspirin.8, 16, 31, 60, 61 However, if atrial fibrillation has developed in the patient, the recommendation is warfarin (INR 2.03.0) or, if anticoagulants cannot be taken, aspirin 325 mg/day.8 Risk factors should be reassessed and managed, with agents and lifestyle changes to control hypertension and dyslipidemia. Antiplatelet agents should be continued in patients with noncardioembolic stroke. Acceptable antiplatelet agents include aspirin (50325 mg/day), aspirin plus extended‐release dipyridamole, and clopidogrel. The combination of aspirin plus extended‐release dipyridamole is suggested over aspirin alone. If the patient cannot tolerate or is allergic to aspirin, clopidogrel is a reasonable alternative.8 The decision of which antiplatelet agent to use should be based on the individual patient's risk factor profile.8 The temptation to put patients on anticoagulation therapy because of a wish to do more should be avoided, as this is likely to expose patients to increased risk without known benefit.60, 61
Consider a common case scenarioa patient with a known history of hypertension and TIA presents with a 30‐minute episode of left arm numbness. The patient has been adherent to his prescribed medications, including aspirin 81 mg/day. What is the appropriate approach to acute treatment at this time? This is a common scenario in emergency departmentsnew‐onset TIA while taking aspirin 81 mg/day. There are advocates for several different treatment regimens in these patients: increasing the aspirin dose to 325 mg/day as a new treatment; discontinuing aspirin and initiating clopidogrel 75 mg/day; discontinuing aspirin 81 mg/day and initiating aspirin 325 mg/day plus clopidogrel 75 mg/day; or discontinuing aspirin 81 mg/day and initiating a combination of aspirin 25 mg plus extended‐release dipyridamole 200 mg twice daily. It is clear that patients with the same disease are treated differently in different institutions. What is the appropriate evidence‐based treatment in this case? The answer is clearno evidence supports increasing the dose of aspirin as a new treatment for this case or initiating aspirin 325 mg/day plus clopidogrel 75 mg/day.16, 17 Based on the literature, for a patient who has recently had another cerebral ischemic event while on treatment, it would make sense to consider switching to another agent. Three agents are recommended by the guidelines: aspirin, clopidogrel, and aspirin plus extended‐release dipyridamole. If treatment 1 were to fail, it would not be against the evidence to initiate treatment 2 or 3.
PATIENTS ON WARFARIN
Data from the Warfarin‐Aspirin Recurrent Stroke Study (WARSS), a large‐scale recurrent stroke prevention trial conducted in 2206 patients, demonstrated that there was no survival benefit for noncardioembolic stroke survivors who were treated with warfarin.60, 61 Yet there are patients still taking warfarin to reduce stroke risk who do not have atrial fibrillation. Unless a patient is allergic to or intolerant of antiplatelet agents such as aspirin, clopidogrel, or dipyridamole, they should not be treated with warfarin for noncardioembolic stroke risk.8 The results of other studies of anticoagulation in recurrent stroke prevention, including the European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT),62 the Stroke Performance for Reporting the Improvement and Translation (SPIRIT) trial,63 and the WASID study,33 have yet to demonstrate a role for warfarin in prevention of noncardioembolic stroke.
Given these trial results, patients currently on warfarin who do not have a cardioembolic risk factor should be placed on antiplatelet therapy with aspirin, aspirin plus extended‐release dipyridamole, or clopidogrel 35 days after discontinuing warfarin therapy. However, it would be advisable to evaluate these patients for atrial fibrillation, as patients with that risk factor should remain on warfarin.8
SUMMARY
In clinical practice, health care providers often must manage patients with complex profiles. Multiple risk factors and comorbidities complicate treatment of these individuals, and robust clinical data are often lacking as clinical trials rarely include such individuals. Guidelines offer recommendations, but these too are often based on extrapolations from clinical trial data. This is particularly true of patients at risk for ischemic stroke, as the primary underlying causevascular diseasehas systemic implications and comorbidities that often complicate treatment.
In general, antiplatelet therapy should be used to prevent recurrent stroke in patients with TIA or noncardioembolic stroke, whereas anticoagulation therapy should be used in patients with cardioembolic stroke such as that caused by atrial fibrillation. However, therapy must be individualized to account for the patient's full risk profile. Conditions such as dyslipidemia and hypertension must be addressed as well, as these not only give rise to stroke but also to the CAD, coronary heart disease, and ACS that may coexist with stroke. Among patients deemed suitable for antiplatelet therapy, class IIa, level A evidence supports the use of aspirin 50325 mg/day, the combination of aspirin and extended‐release dipyridamole, and clopidogrel for secondary prevention of stroke.8
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
- ,,,,.Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack.Stroke.2005;36:1285–1287.
- ,.Underestimation of the early risk of recurrent stroke.Stroke.2004;35:1925–1929.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,,.Very early risk of stroke after a first transient ischemic attack.Stroke.2003;34:e138–e142.
- ,,, et al.Occurrence of secondary ischemic events among persons with atherosclerotic vascular disease.Stroke.2002;33:901–906.
- .Secondary prevention of stroke and transient ischemic attack.Circulation.2007;115:1615–1621.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:483S–512S.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke.Circulation.2006;113:409–449.
- Trends in Cardiovascular operations and procedures. US 1979‐2002. Available at: http://iis‐db.stanford.edu/evnts/4748/DenaBravata_MarkHlatky_RIP.PPT#258,4,Prevalence.Accessed September 10, 2007.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J.2005;26:804–847.
- American Heart Association. Stent Procedure. Available at: http://www.americanheart.org/presenter.jhtml?identifier= 4721. Accessed September 10, 2007.
- .Drug‐eluting coronary stents—a note of caution.Med J Aust.2007;186:253–255. Available at: http://www.mja.com.au/public/issues/186_05_050307/har10076_fm. html. Accessed September 10, 2007.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet.2001;358:527–533.
- .Long‐term clopidogrel therapy after percutaneous coronary intervention in PCI‐CURE and CREDO: the “Emperor's New Clothes” revisited.Eur Heart J.2004;25:720–722.
- .Long‐term clopidogrel therapy in the drug‐eluting stent era: beyond CREDO and PCI‐CURE.Eur Heart J.2004;25:1364.
- ,,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,,, et al. CHARISMA Investigators.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354:1706–1717.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115:813–818.
- ,,.Stroke in patients with heart failure and reduced left ventricular ejection fraction.Neurology.2000;54:288–294.
- ,,, et al.Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial: the SAVE Investigators.N Engl J Med.1992;327:669–677.
- ,,, et al.Ventricular dysfunction and the risk of stroke after myocardial infraction.N Engl J Med.1997;336:251–257.
- ,,,.Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials.J Am Coll Cardiol.1997;29:1074–1080.
- ,,,.Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study.Neurology.1994;44:626–634.
- ,,.Pharmacological prevention of thromboembolism in patients with left ventricular dysfunction.Am J Cardiovasc Drugs.2006;6:41–49.
- The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.N Engl J Med.1991;1325:293–302.
- ,,,,,.Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Trial.J Am Coll Cardiol.1998;31:419–425.
- ,,, et al.The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure.Am Heart J.2004;148:157–164.
- ,,, et al.The Warfarin and Antiplatelet Therapy in Heart Failure trial (WATCH): rationale, design, and baseline patient characteristics.J Card Fail.2004;10:101–112.
- . The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial: a report on a presentation at the late‐breaking clinical trials session of the 53rd Annual Scientific Session of the American College of Cardiology; March 7‐10, 2004; New Orleans (LA). Available at: http://www.cardiologyupdate.org/crus/402‐033.pdf. Accessed September 10, 2007.
- ,,, et al, on behalf of the WARCEF Investigators.Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design.J Card Fail.2006;12:39–46.
- ,,,.Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study.Stroke.1995;26:14–20.
- Warfarin‐Aspirin Symptomatic Intracranial Disease (WASID) Study Group.Prognosis of patients with symptomatic vertebral or basilar artery stenosis.Stroke.1998;29:1389–1392.
- ,,, et al.Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis.N Engl J Med.2005;352:1305–1316.
- .Warfarin, aspirin, and intracranial vascular disease.N Engl J Med.2005;352:1368–1370.
- ,,, et al. Primary prevention of ischemic stroke.A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.Circulation.2006;113:e873–e923.
- ,,, et al.Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. CHS Collaborative Research Group.Stroke.1992;23:1752–1760.
- Medical Research Council Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group.Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial.Lancet.2004;363:1491–1502.
- ,,, et al.Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis.Lancet.2003;361:107–116.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.N Engl J Med.1998;339:1415–1425.
- ,,, et al.The North American Symptomatic Carotid Endarterectomy Trial. Surgical results in 1415 patients.Stroke.1999;30:1751–1758.
- ,,,,.Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke.Stroke.2004;35:2855–2861.
- CAVATAS Investigators.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial.Lancet.2001;357:1729–1737.
- ,,, et al.Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis.N Engl J Med.2006;355:1660–1671.
- ,,; SPACE Collaborative Group.30 Day results from the SPACE trial of stent‐protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non‐inferiority trial.Lancet.2006;368:1239–1247.
- The French Study of Aortic Plaques in Stroke Group.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke.N Engl J Med.1996;334:1216–1221.
- ,,.Protruding atheromas in the thoracic aorta and systemic embolization.Ann Intern Med.1991;115:423–427.
- ,,,.Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants.J Am Coll Cardiol.1999;33:1317–1322.
- ,,,.Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke.J Am Coll Cardiol.1998;31:134–138.
- ,, et al.Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque.Am J Cardiol.2002;90:1320–1325.
- ,,, et al.Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis.Stroke.2005;36:2286–2288.
- ,,, et al.Association between platelet receptor occupancy after eptifibatide (Integrilin) therapy and patency, myocardial perfusion, and ST‐segment resolution among patients with ST‐segment‐elevation myocardial infarction. An INTEGRITI (Integrilin and Tenecteplase in Acute Myocardial Infarction) Substudy.Circulation.2004;110:679–684.
- ,,, et al.Prehospital therapy with the platelet glycoprotein IIb/IIIa inhibitor eptifibatide in patients with suspected acute coronary syndromes. The Bochum Feasibility Study.Chest.2004;126:935–941.
- ,.Diagnosis and management of ST elevation myocardial infarction: a review of the recent literature and practice guidelines.Mt Sinai J Med.2006;73:469–481.
- ,.Unstable angina and non‐ST‐segment myocardial infarction: an evidence‐based approach to management.Mt Sinai J Med.2006;73:449–468.
- ,,, et al.Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association.Stroke.2003;34:2310–2322.
- ,,.Evolving perspectives on clopidogrel in the treatment of ischemic stroke.JCardiovasc Pharmacol Ther.2006;11:245–248.
- ,,,,,.European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143:1–13.
- .Secondary stroke prevention with antiplatelet therapy with emphasis on the cardiac patient.J Am Coll Cardiol.2005;46:752–755.
- CAPRIE Steering Committee.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).Lancet.1996;348:1329–1339.
- .Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. Is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?Stroke.2002;33:1723–1726.
- ,,, et al.Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study.Cerebrovasc Dis.2006;22:4–12.
- ESPRIT Study Group.Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial.Lancet Neurol.2007;6:115–124.
- Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group.A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin.Ann Neurol.1997;42:857–865.
Copyright © 2008 Society of Hospital Medicine
Hospitalist Role in Stroke Prevention
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11
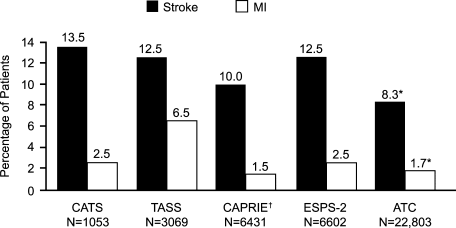
MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11

MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
Each year in the United States 700,000 individuals experience a stroke500,000 of them for the first time. Despite advances in stroke prevention, this number has increased dramatically over the last quarter century.1 Between 1979 and 2004, the annual number of hospital discharges with stroke as a primary diagnosis swelled to 906,000, a 21% increase over the rate in 1979.1 In the next 1015 years, this number is predicted to double in parallel with a doubling of the number of Americans older than age 65 years. Mortality from stroke is projected to increase faster than the overall US population.2 In addition, the prevalence of diabetes, a major ischemic stroke risk factor, is increasing at an alarming rate.1 A second major risk factor, hypertension, also occurs more frequently in older people and thus is expected to increase in prevalence over the next few decades.1, 3 Blacks, Hispanics, and Mexican Americans, growing segments of the US population, are disproportionately affected by stroke.1
The impact of stroke extends far beyond the initial episode. Stroke is a leading cause of long‐term disability in the United States.1 Total estimated cost for stroke care in 2007 is $62.7 billion. Prevention is the key to reducing the grave personal and societal burden of this condition.
Efforts to prevent the approximately 200,000 recurrent strokes that occur each year are critical. Stroke itself is a harbinger of future stroke, and secondary strokes are frequently more severe and disabling.4 Numerous studies have found that among stroke patients, recurrent stroke is the most likely secondary cardiovascular event, particularly in the first few months following the index event (only in the first 3 months, however; then death from cardiac disease becomes more important; Fig. 1).5, 6 Transient ischemic attack (TIA), once considered a relatively benign event, is now recognized as a significant risk factor for stroke.7, 8 A recent study suggests that 1 in 10 TIA patients will have a stroke in the 90 days after the event, and 24% of those strokes will occur within 48 hours.8 Moreover, improved imaging techniques have revealed that even patients with resolution of symptoms within 1 hour may have evidence of infarction.9, 10 The longer the duration of symptoms, the greater the probability of infarction detectable with magnetic resonance imaging.9, 10 Because the greatest risk of recurrent stroke occurs within hours of the first event, secondary prevention must be initiated as soon as possible after diagnosis.11

MANAGEMENT OF ACUTE STROKE BY HOSPITALISTS
Stroke care is a rapidly evolving field in which expeditious and careful inpatient care significantly affects outcome. Hospitalists are in a unique position to improve acute stroke care and initiate secondary stroke prevention in several ways. First, there is a shortage of neurologists to care for patients with stroke. In one survey of Medicare data from 1991, prior to the widespread presence of hospitalists, only 1 in 9 stroke patients (11%) had a neurologist as the attending physician.12 At that time, there were only 3.25 nonfederal patient care neurologists per 100,000 population. Although the ratio may have improved somewhat in the intervening years (there were an estimated 5.3 self‐reported neurologists per 100,000 population as of 2005),13 the limited number of neurologists combined with the increasing incidence of stroke is expected to reduce the fraction of stroke patients having a neurologist involved in their care. Because neurology practices tend to be concentrated in urban areas, the shortage is likely to affect nonurban areas to a greater degree. The number of hospitalists, currently estimated to be 20,000 in the United States, is projected to reach 30,000 by 2010.14 In the simplest terms, hospitalists are the logical choice to fill the need for physicians to manage inpatient stroke.
Perhaps the most compelling reason for hospitalists to be involved in the care of stroke patients is clinical: patients with stroke frequently have multiple comorbid conditions that affect outcomes and are not within the traditional purview of neurology. A retrospective analysis of data from 1802 patients seen in a geriatric practice revealed that 56% of patients with stroke also had coronary artery disease, and 28% had peripheral arterial disease.15 In addition, the major risk factors for strokediabetes and hypertensionwould be expected to be prevalent in this population. Timely and effective management can improve secondary stroke prevention as well as prevent exacerbation of existing conditions.
A recent report compared outcomes in 44,099 patients following stroke according to physician specialty.16 Although patients treated by neurologists alone had a 10% lower risk of 30‐day mortality compared with those treated by generalists (family practice physicians, general practitioners, or internists) despite having more severe stroke, collaborative care reduced that risk an additional 6%.16 The risk of rehospitalization for infections and aspiration pneumonia within 30 days was 12% lower for those treated by neurologists. However, these patients had a significant, 17% increased relative risk of rehospitalization for coronary heart disease (95% confidence interval [CI], 1.021.34).16
Comanagement of stroke patients by hospitalists and neurologists is likely to become more common over time, as proposed by Likosky and Amin.17 Although studies have not specifically compared outcomes in patients with stroke who have been treated by hospitalists versus other types of physicians, implementation of hospitalist services has been associated with improved short‐term mortality and rehospitalization rates compared with traditional care.1820 Approximately 85% of hospitalists are trained in internal medicine.21 In addition, they have skill sets focusing on the specialized needs of inpatients. As hospitalists assume a greater role in the management of stroke, research into the benefits of collaborative care can be explored.
Finally, hospitalists are ideally positioned to champion the use of standardized protocols for secondary stroke prevention at their institutions. Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry showed that a minority of acute stroke patients are treated according to established guidelines.22 The 4 prototype registries were in Georgia, Massachusetts, Michigan, and Ohio. The percentage of relevant patient populations that had lipid profiles assessed ranged between 28% and 34%. For smoking‐cessation education, the range was between 17% and 34%. Anticoagulant prescribing for relevant populations at discharge ranged from 64% to 90%, and antithrombotic prescribing ranged from 88% to 98%.22
The use of protocols that initiate secondary prevention of cerebrovascular and cardiovascular events has been demonstrated to improve patient adherence to evidence‐based treatment after discharge.2328 The Preventing Recurrence of Thromboembolic Events Through Coordinated Treatment (PROTECT) program was designed to integrate secondary stroke prevention measures into the standard stroke care provided during acute hospitalization (Table 1).26 Use of appropriate antithrombotic medication was achieved in 100% of cases. Use of statins, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and thiazide diuretics improved significantly during the first year of implementation (P < .001). Patient education in all 4 of the areas established was carried out in 100% of patients prior to discharge.26 Tools for establishing similar hospital‐based secondary prevention programs are presently available from the University of California at Los Angeles PROTECT Program and other programs.
|
| Initiation and maintenance of appropriate: |
| 1.Antithrombotic therapy |
| 2.Statin therapy |
| 3.Angiotensin‐converting enzyme or angiotensin receptor blocker therapy |
| 4.Thiazide diuretic therapy |
| 5.Smoking‐cessation advice and referral to a formal cessation program |
| 6.American Heart Association diet |
| 7.Exercise counseling |
| 8.Stroke education, including knowledge of stroke warning signs and need to call 911 in the event of a cerebrovascular event, as well as awareness of individual's own risk factors |
An essential part of any effort to develop standardized treatment procedures must include a plan to minimize any discontinuity of care after discharge. Standardized procedures need to be implemented to ensure communication of discharge summaries to outpatient clinicians in a timely and complete fashion. Only 19% of 226 outpatient physicians responding to a recent survey were satisfied or very satisfied with the timeliness of discharge summaries they received for their patients.29 Approximately one third of respondents reported that most of their patients (60%) were seen for their follow‐up outpatient visit before discharge summaries had been received. Only about one third (32%) of the respondents were satisfied or very satisfied with the summary content. Forty‐one percent believed that at least 1 of their patients hospitalized in the previous 6 months had experienced an adverse event that could have been prevented with improved transfer of discharge information.29
Development of electronic discharge summaries is an obvious alternative to conventional paper versions. This area has received less attention than others that more directly affect patient care. As the primary inpatient physicians, hospitalists can effectively implement improvements in communication among hospital staff and outpatient health care providers.
SUMMARY
This supplement is a call to action for hospitalists based on a roundtable discussion conducted in March 2007. Participants included hospitalists, neurohospitalists, vascular neurologists, and neurointensivists. The objectives of the meeting were to review the clinical data supporting current practice guidelines for secondary prevention of noncardioemboic ischemic stroke, to develop best‐practice recommendations for hospitalist‐based care of stroke inpatients, and finally to recommend improvements in transfer of information to outpatient health care providers.
The consensus of the participants is reported in the following 3 articles. The first, Evidence‐based Medicine: Review of Guidelines and Trials in Prevention of Secondary Stroke, includes an overview of the pathophysiology of stroke and TIA and reviews the clinical data supporting current treatment guidelines. Several case studies illustrating challenging or difficult aspects of secondary stroke prevention are presented in the second article, Secondary Prevention of Ischemic Stroke: Challenging Patient Scenarios. These cases focus on commonly encountered difficulties for which there may not be clear evidence or consensus. In the final article, Systems Approach to Standardization of Care in the Secondary Prevention of Noncardioembolic Ischemic Stroke, the best‐practices recommendations developed at the roundtable are presented. The role of the hospitalist in long‐term prevention strategies and the effective transfer of care to outpatient providers are discussed.
As the hospitalist movement grows, hospital‐based physicians need to identify opportunities to use their unique skills. By taking the lead in improving processes that result in better patient outcomes, hospitalists can ensure that the value of this nascent field will continue to gain recognition in the broader, sometimes skeptical medical community. We sincerely hope that you agree that integrating secondary prevention into inpatient acute stroke care is just such an opportunity. Furthermore, we hope the information we have provided will be useful to you in your hospital‐based practice.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
- ,,, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Heart Disease and Stroke Statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2007;115:e69–e171.
- ,.Thirty‐year projections for deaths from ischemic stroke in the United States.Stroke.2003;34:2109–2112.
- ,,,,,.Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study.Stroke.2006;37:2493–2498.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .Choice of endpoints in antiplatelet trials: which outcomes are most relevant to stroke patients?Neurology.2000;54:1022–1028.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomized trials of antiplatelet therapy for prevention of deathmyocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,.Timing of TIAs preceding stroke: time window for prevention is very short.Neurology.2005;64:817–820.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Diffusion MRI in patients with transient ischemic attacks.Stroke.1999;30:1174–1180.
- ,,,,,.Diffusion‐weighted MR imaging in the acute phase of transient ischemic attacks.AJNR Am J Neuroradiol.2002;23:77–83.
- .The emergency department: first line of defense in preventing secondary stroke.Acad Emerg Med.2006;13:215–222.
- ,,,,,.What role do neurologists play in determining the costs and outcomes of stroke patients?Stroke.1996;27:1937–1943.
- ,. Member Demographics Subcommittee of American Academy of Neurology.Neurologists 2004.St. Paul, MN:American Academy of Neurology;2005.
- Society of Hospital Medicine. Hospital medicine market profile. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Publications/TheHospitalist/Market_ Profile.pdf. Accessed August 30, 2007.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,,.30‐Day survival and rehospitalization for stroke patients according to physician specialty.Cerebrovasc Dis.2006;22:21–26.
- ,.Who will care for our hospitalized patients?Stroke.2005;36:1113–1114.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,.A comparison of two hospitalist models with traditional care in a community teaching hospital.Am J Med.2005;118:536–543.
- Society for Hospital Medicine. Definition of a hospitalist. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/AboutSHM/DefinitionofaHospitalist/Definition_of_a_Hosp.htm. Accessed August 30, 2007.
- ;for the Paul Coverdell Prototype Registries Writing Group.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;3:1232–1240.
- ,.Stroke best practices: a team approach to evidence‐based care.J Natl Med Assoc.2004;96:5S–20S.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,, et al.PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events.Neurology.2004;63:1217–1222.
- American Stroke Association. Get with the Guidelines. Available at: http://www.strokeassociation.org/presenter.jhtml?identifier=3002728 ‐ 39k. Accessed April 11, 2007.
- UCLA Stroke PROTECT Program. Available at: http://strokeprotect.mednet.ucla.edu. Accessed April 11, 2007.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1:317–320.
Copyright © 2008 Society of Hospital Medicine