User login
Worsening nausea, vomiting, and dizziness • 20-pound weight loss in 2 months • mild hearing loss • reoccurring episodes of falls • Dx?
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
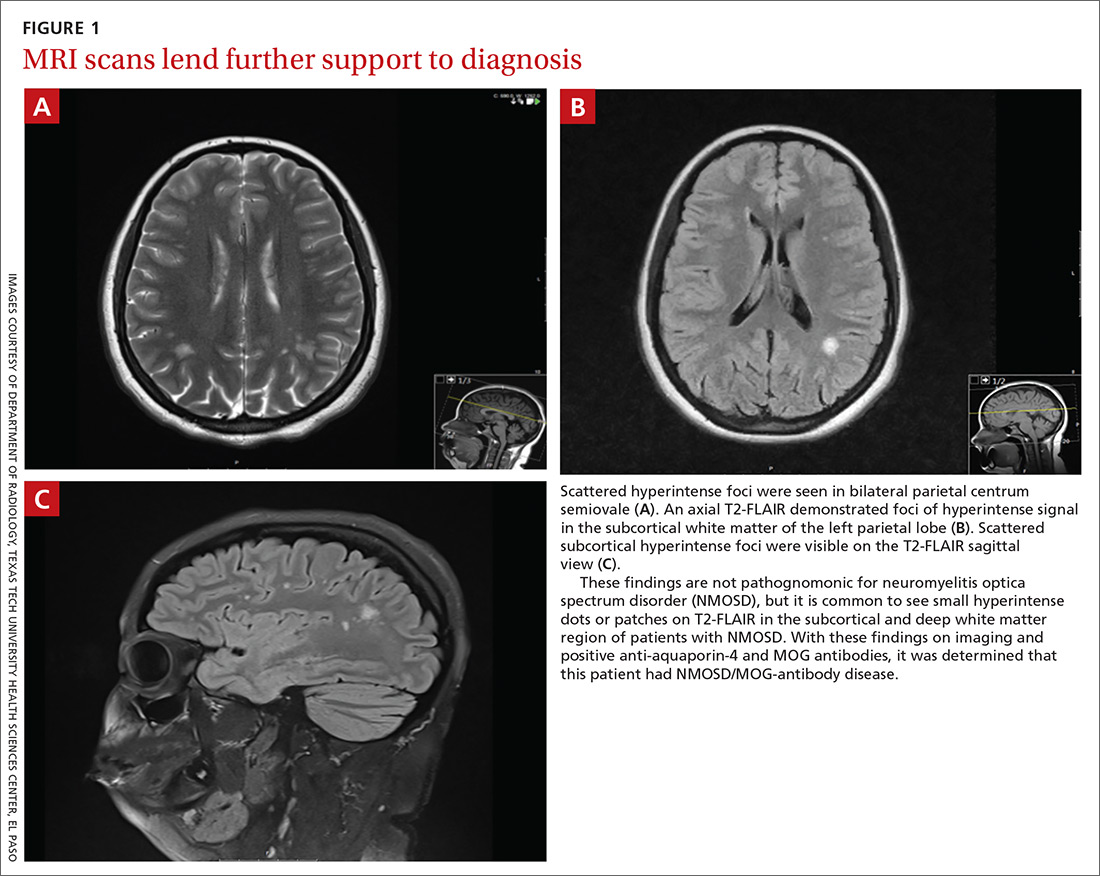
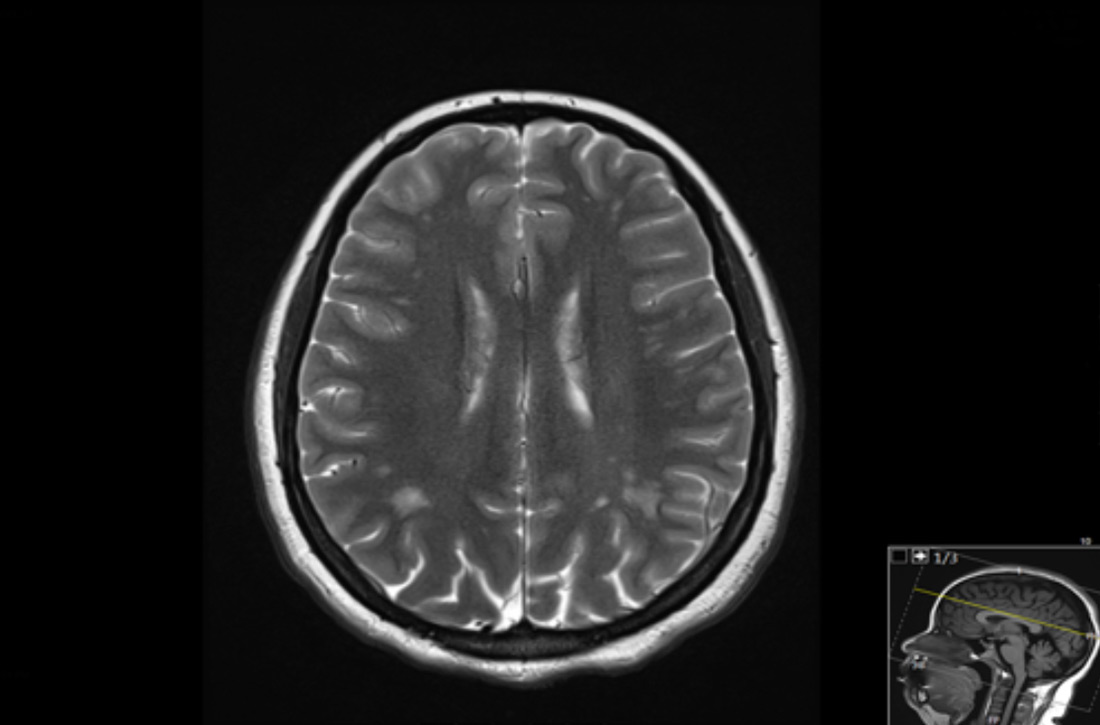
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
THE CASE
A 26-year-old Hispanic/African American woman presented to our clinic with a 2-month history of nausea and vomiting, along with dizziness. The nausea and vomiting persistently worsened, and she was only able to tolerate apples and berries. During this 2-month period, she lost 20 pounds and her symptoms progressed to include pruritus, ataxia, and mild hearing loss, with reoccurring episodes of falls.
THE DIAGNOSIS
On examination, she was found to be bradycardic with a heart rate of 47 beats/min, right- axis deviation, and inverted T waves in leads I, II, and augmented vector left. Her family history included the death of an aunt who was in her early 30s due to an unknown heart condition.
Echocardiogram identified mild mitral valve regurgitation with an ejection fraction of 55% to 60% (reference range: 55%-70%). Cardiology determined that her bradycardia was not the source of her symptoms. A neurologic exam identified 3+ hyperreflexia (indicating the reflex was increased), tandem gait instability, and left oculomotor dysfunction.
Brain magnetic resonance imaging (MRI) identified bilateral parietal white matter lesions where a demyelinating process could not be excluded (FIGURE 1A). The patient’s symptoms of nausea and vomiting continued, and she only tolerated peanuts and liquids. An MRI of the spine was negative.
Laboratory testing revealed that the patient was negative for human immunodeficiency virus (HIV), syphilis, Lyme disease, and lupus. Her thyroid-stimulating hormone level was 1.7 mIU/L (reference range: 0.4-4.2 mIU/L), and her vitamin B12 level was 504 pg/mL (reference range: 160-950 pg/mL).
The patient’s lumbar puncture was negative for oligoclonal bands. The IgG synthesis rate/index cerebrospinal fluid (CSF) was –3.9, ruling out multiple sclerosis. Her CSF culture was negative, with a glucose level of 42 mg/dL (reference range: 70-110 mg/dL), colorless appearance, 1 white blood cell, and spinal albumin of 12.2 mg/dL (reference range: 8-42 mg/dL). The visual evoked potential was negative. The aquaporin-4 (AQP4) antibody was positive at 3.4 U/mL, and the myelin oligodendrocyte glycoprotein (MOG) antibody was positive.
Gastroenterology concluded a normal gastric accommodation and unremarkable computed tomography (CT) enterography. Moderate erosions were identified in the stomach with an erythematous gastropathy. The patient was placed on a proton pump inhibitor.
Continue to: Following the examination...
Following the examination and laboratory testing, the patient was admitted under our family medicine service for neuromyelitis optica (NMO) affecting the area postrema. NMO, also known as Devic’s disease, is an autoimmune disorder that affects the spinal cord and optic nerves. Autoantibodies against AQP4 are created in the periphery and are directed against astrocytes in the central nervous system. These antibodies bind to the foot processes of astrocytes, inducing complement-mediated cell damage and granulocyte infiltration.1-5
Intravenous methylprednisolone was initiated at 250 mg every 6 hours for 3 days. A repeat brain MRI demonstrated nonspecific multiple scattered foci of hyperintense signal involving the subcortical supratentorial white matter without abnormal enhancement, most likely representing nonactive demyelinating plaques (FIGURES 1B and 1C).
Dx is revisited. Our patient was referred to an NMO clinic for evaluation. After further testing (including a repeat MRI based on the neurologist’s specifications, anti-aquaporin antibody testing, and MOG-antibody testing) and case discussion, it was determined that the patient had MOG-antibody disease. This disease, along with NMO, comprise a spectrum of disorders referred to as neuromyelitis optica spectrum disorder (NMOSD).
The patient was subsequently prescribed a rituximab infusion, 500 mg/50 mL, to treat the current attack. One infusion was to be completed weekly for 2 weeks with plans to repeat treatment every 6 months to prevent flares of NMO. During the first dose, the patient had a reaction to the treatment, which caused pruritus and chest tightness. She was able to complete the infusion after being treated with diphenhydramine.
Tx continued. In order to complete the second of 2 infusions of rituximab, the patient was pretreated with oral methylprednisolone the night before the infusion, along with diphenhydramine and acetaminophen on the day of treatment. Fortunately, the patient tolerated the infusion well with no adverse effects or reactions.
Continue to: DISCUSSION
DISCUSSION
Within the NMO spectrum, the MOG antibody is positive in up to 42% of AQP4-seronegative cases.6 MOG is a minor myelin component that is expressed exclusively in the central nervous system on the surface of myelin and oligodendrocyte processes. The role of this glycoprotein is not well understood but is hypothesized to function as a cell surface receptor or cell adhesion molecule.7
Among a cohort of 252 patients from the United Kingdom who tested positive for the MOG-IgG1 antibody, optic neuritis was seen in 55%, while 18% experienced transverse myelitis, and 15% had a history of area postrema syndrome. A brain MRI identified lesions in all areas of the brain including the brain stem, cerebellum, and cerebral hemispheres.8
Risk factors for NMOSD include female gender, Asian and African ethnicities, Epstein Barr virus seropositivity, and tobacco abuse.
Differential diagnosis. Many diseases or conditions that are inflammatory, autoimmune, infectious, or neoplastic can involve the central nervous system and mimic the clinical and radiologic phenotypes of NMOSD-AQP4. They include lupus, SjÖgren’s syndrome, multiple sclerosis, sarcoidosis, acute disseminated encephalomyelitis, HIV, and vitamin B12 deficiency.
Treatment. The standard treatment is intravenous methylprednisolone, 1 g/d for 3 to 5 days followed by a steroid taper. Therapeutic plasma exchange is recommended for refractory cases and in patients with spinal cord demyelination.9-11 Rituximab is the first-line therapy for attack prevention12-15 in NMOSD broadly and may be effective in MOG antibody disease, as well. In an open-label study of patients with NMOSD treated with rituximab, 64% were relapse free at follow-up, which ranged from 12 to 67 months.13 In a long-term study of patients treated with rituximab, 87% maintained a reduced relapse rate and 93% had improvement or stability over a 5-year follow-up.14
Continue to: Our patient
Our patient. After her diagnosis of NMOSD/MOG-antibody disease, our patient’s symptoms progressed to include vertigo, vestibular ataxia, pruritus, left foot drop, lower extremity numbness, and decreased hearing. After the second rituximab infusion her symptoms continued, but over time stabilized and have not worsened. She currently receives gabapentin 300 mg every 8 hours, as needed, for extremity numbness (which has been working well) along with sertraline 100 mg/d for depression.
Subsequent office visits have showed no further weight loss. Based on the current response to the rituximab, her prognosis is undetermined by Neurology as they continue to monitor for progression.
THE TAKEAWAY
Vestibular ataxia, foot drop, pruritus, vertigo, decreased hearing, numbness, and oculomotor dysfunction in the presence of nausea and vomiting should raise suspicion for NMOSD. The presence of AQP4 antibodies along with demyelinating central nervous system lesions, is highly indicative of NMO. The presence of MOG antibodies may indicate NMOSD/MOG-antibody disease. The initial treatment of NMOSD is intravenous methylprednisolone, which can be followed by treatment with rituximab to achieve remission.
CORRESPONDENCE
Daniel Murphy, MD, FAAFP, Department of Family and Community Medicine, Texas Tech University Health Science Center El Paso, 9849 Kenworthy Street, El Paso, Texas 79924; Daniel.murphy@ttuhsc.edu
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.
1. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221-2231.
2. Ratelade J, Zhang H, Saadoun S, et al. Neuromyelitis optica IgG and natural killer cells Produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123:861-872.
3. Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349-361.
4. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titer. Brain. 2007;130:1235-1243.
5. Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072-3080.
6. Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are Mog-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017; 264:2088-2094.
7. Peschl P, Bradi M, Hoftberger R, et al. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529.
8. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128-3138.
9. Sellner J, Boggild M, Clanet M, et al. EFNS Guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019-1032.
10. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206-216.
11. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128-132.
12. Collongues N, Brassat D, Maillart E, et al. Efficacy of rituximab in refractory neuromyelitis optica. Mult Scler. 2016;22:955-959.
13. Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord. 2016;9:180-188.
14. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-1117.
15. Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-1420.