User login
Letter to the Editor
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
Novel Configuration of a Traditional RRT
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
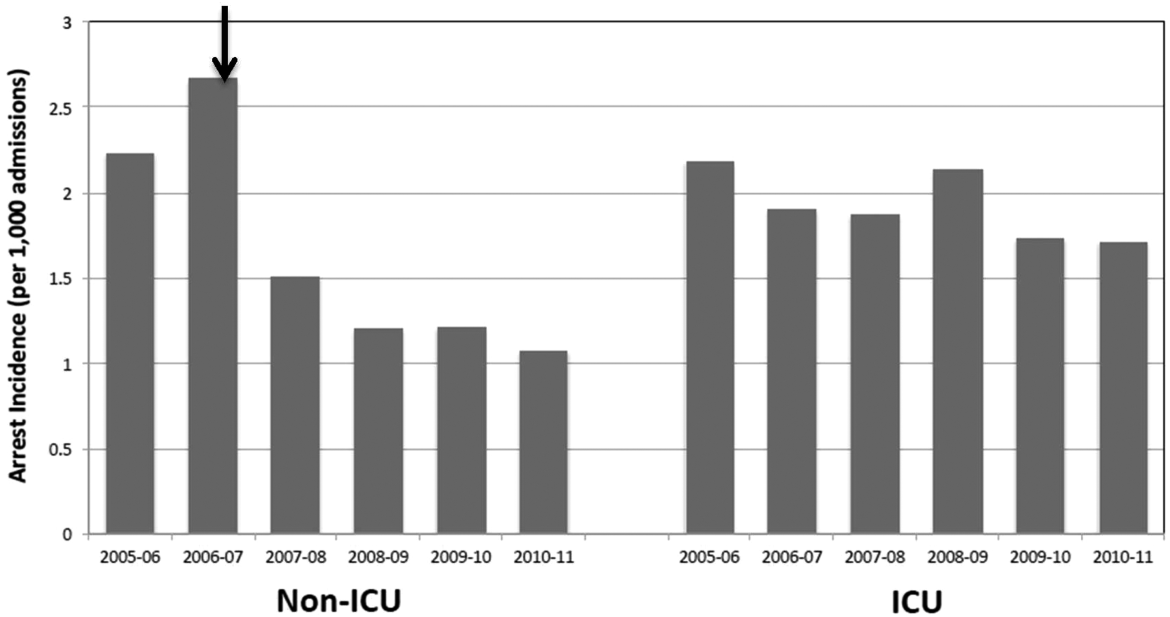
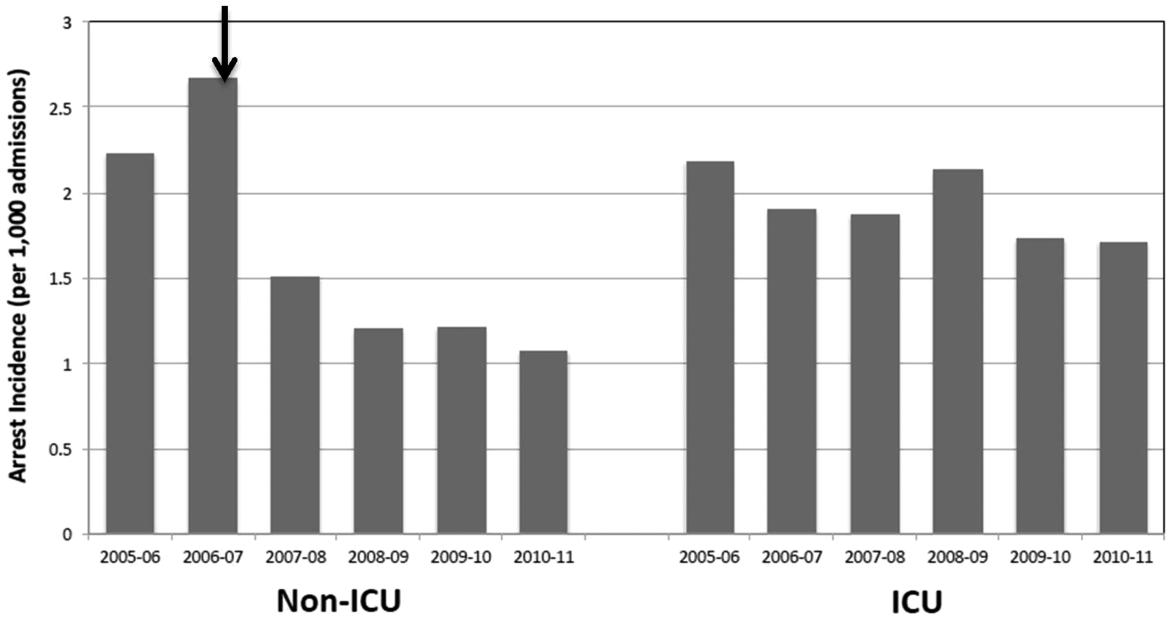
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).

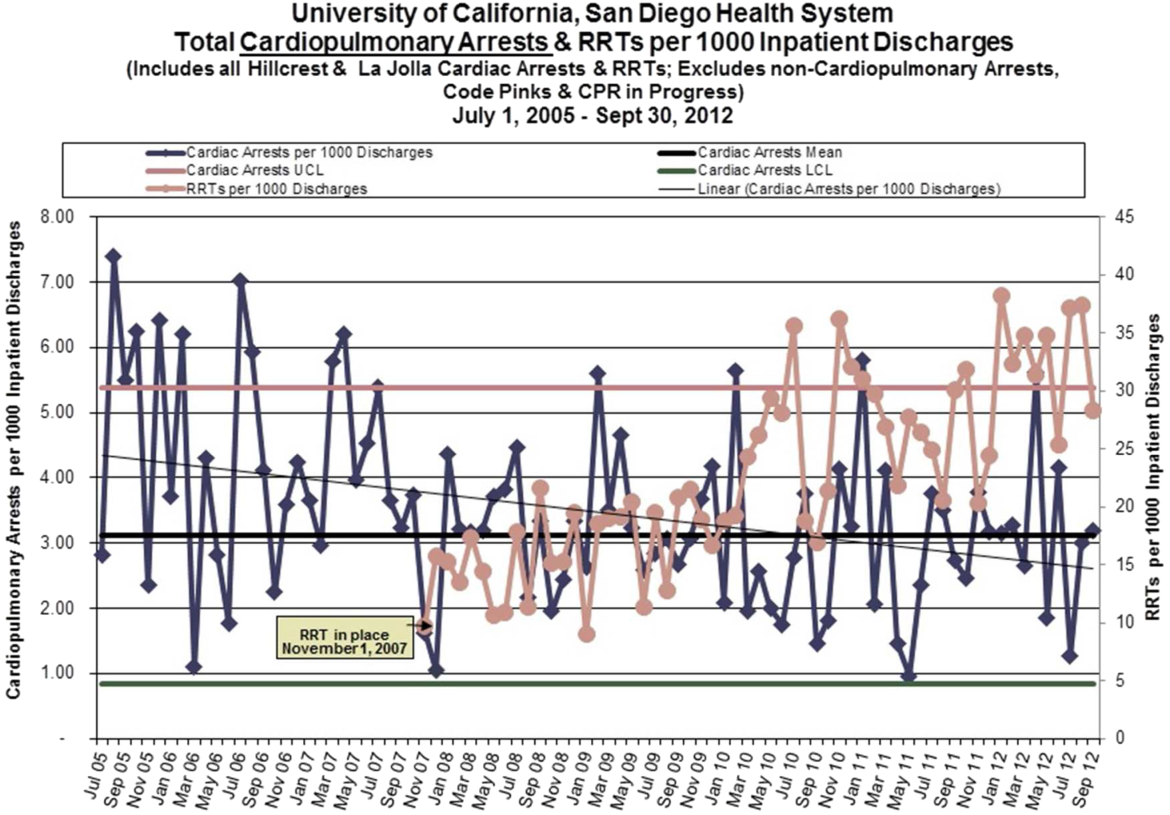
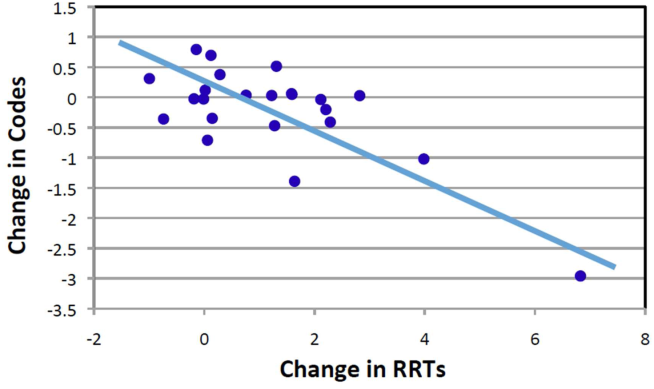
Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).
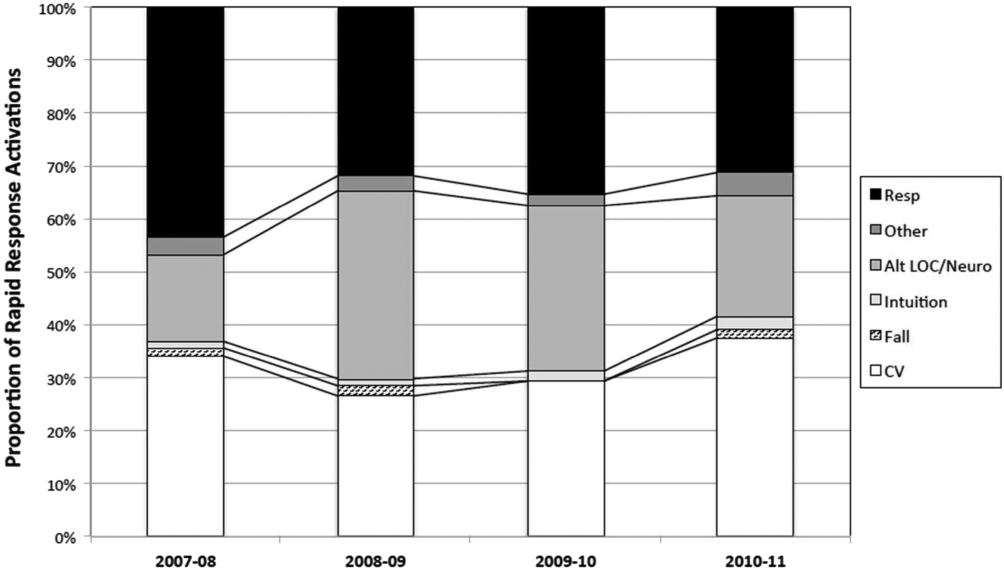
The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.
DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).


Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).


The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.

DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).


Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).


The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.

DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
© 2015 Society of Hospital Medicine
In‐hospital CPR Practices
An estimated 200,000 adult patients suffer cardiac arrest in US hospitals each year, of which <20% survive to hospital discharge.[1, 2] Patient survival from in‐hospital cardiac arrest (IHCA), however, varies widely across hospitals, and may be partly attributed to differences in hospital practices.[3, 4, 5] Although there are data to support specific patient‐level practices in the hospital, such as delivery of electrical shock for ventricular fibrillation within 2 minutes of onset of the lethal rhythm,[6] little is known about in‐hospital systems‐level factors. Similar to patient‐level practices, some organizational and systems level practices are supported by international consensus and guideline recommendations.[7, 8] However, the adoption of these practices is poorly understood. As such, we sought to gain a better understanding of current US hospital practices with regard to IHCA and resuscitation with the hopes of identifying potential targets for improvement in quality and outcomes.
METHODS
We conducted a nationally representative mail survey between May 2011 and November 2011, targeting a stratified random sample of 1000 hospitals. We utilized the US Acute‐Care Hospitals (FY2008) database from the American Hospital Association to determine the total population of 3809 community hospitals (ie, nonfederal government, nonpsychiatric, and nonlong‐term care hospitals).[9] This included general medical and surgical, surgical, cancer, heart, orthopedic, and children's hospitals. These hospitals were stratified into tertiles by annual in‐patient days and teaching status (major, minor, nonteaching), from which our sample was randomly selected (Table 1). We identified each hospital's cardiopulmonary resuscitation (CPR) committee (sometimes known as code committee, code blue committee, or cardiac arrest committee) chair or chief medical/quality officer, to whom the paper‐based survey was addressed, with instructions to forward to the most appropriate person if someone other than the recipient. This study was evaluated by the University of Chicago institutional review board and deemed exempt from further review.
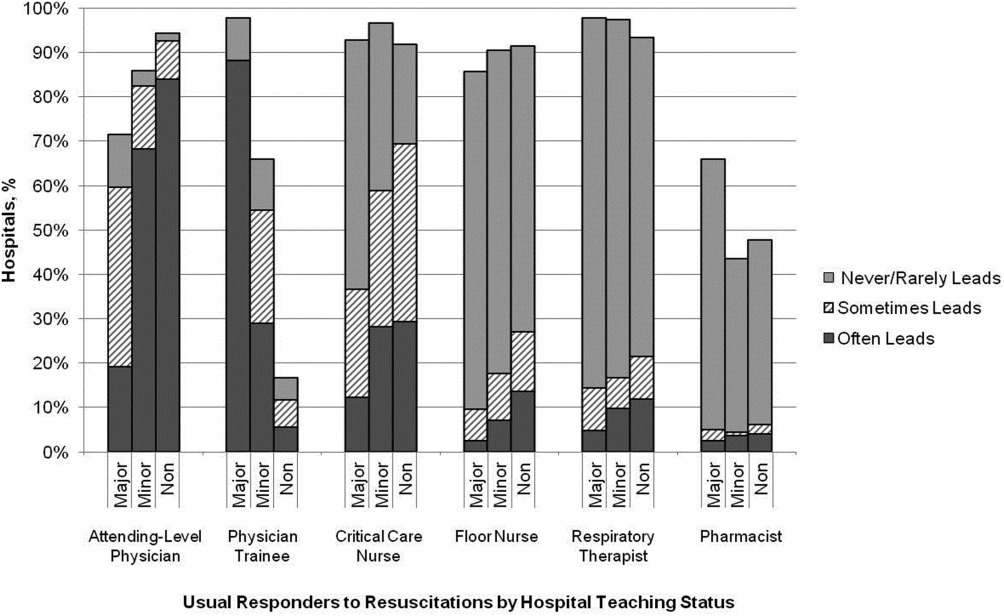
Survey
The survey content was developed by the study investigators and iteratively adapted by consensus and beta testing to require approximately 10 minutes to complete. Questions were edited and formatted by the University of Chicago Survey Lab (Chicago, IL) to be more precise and generalizable. Surveys were mailed in May 2011 and resent twice to nonresponders. A $10 incentive was included in the second mailing. When more than 1 response from a hospital was received, the more complete survey was used, or if equally complete, the responses were combined. All printing, mailing, receipt control, and data entry were performed by the University of Chicago Survey Lab, and data entry was double‐keyed to ensure accuracy.
Response rate was calculated based on the American Association for Public Opinion Research standard response rate formula.[10] It was assumed that the portion of nonresponding cases were ineligible at the same rate of cases for which eligibility was determined. A survey was considered complete if at least 75% of individual questions contained a valid response, partially complete if at least 40% but less than 75% of questions contained a valid response, and a nonresponse if less than 40% was completed. Nonresponses were excluded from the analysis.
Statistical Analysis
Analyses were performed using a statistical software application (Stata version 11.0; StataCorp, College Station, TX). Descriptive statistics were calculated and presented as number (%) or median (interquartile range). A [2] statistic was used to assess bias in response rate. We determined a priori 2 indicators of resource allocation (availability of a CPR committee and dedicated personnel for resuscitation quality improvement) and tested their association with quality improvement initiatives, using logistic regression to adjust for hospital teaching status and number of admissions as potential confounders. All tests of significance used a 2‐sided P<0.05.
RESULTS
Responses were received from 439 hospitals (425 complete and 14 partially complete), yielding a response rate of 44%. One subject ID was removed from the survey and could not be identified, so it was excluded from any analyses. Hospital demographics were similar between responders and nonresponders (P=0.50) (Table 1). Respondents who filled out the surveys included chief medical/quality officers (n=143 [33%]), chairs of CPR committees (n=64 [15%]), members of CPR committees (n=29 [7%]), chiefs of staff (n=33 [8%]), resuscitation officers/nurses (n=27 [6%]), chief nursing officers (n=13 [3%]), and others (n=131 [30%]).
| Teaching Status | Annual Inpatient Days | Total | ||
|---|---|---|---|---|
| <17,695 | 17,695‐52,500 | >52,500 | ||
| ||||
| Major | 1/2 (50) | 1/8 (13) | 40/82 (49) | 42/92 (46) |
| Minor | 13/39 (33) | 40/89 (45) | 62/133 (47) | 115/261 (44) |
| Nonteaching | 141/293 (48) | 100/236 (42) | 40/118 (34) | 281/647 (43) |
| Total | 156/335 (47) | 143/335 (43) | 145/336 (43) | 438/1,000 (44) |
Table 2 summarizes structure, equipment, quality improvement, and pre‐ and postarrest practices across the hospitals. Of note, 77% of hospitals (n=334) reported having a predesignated, dedicated code team, and 66% (n=281) reported standardized defibrillator make and model throughout their hospital. However, less than one‐third of hospitals utilized any CPR assist technology (eg, CPR quality sensor or mechanical CPR device). The majority of hospitals reported having a rapid response team (RRT) (n=391 [91%]). Although a therapeutic hypothermia protocol for postarrest care was in place in over half of hospitals (n=252 [58%]), utilization of hypothermia for patients with return of spontaneous circulation was infrequent.
| Value | 2010 AHA Guidelines | |
|---|---|---|
| ||
| Structure | ||
| Existing CPR committee | 270 (66) | |
| CPR chair | ||
| Physician only | 129 (48) | |
| Nurse only | 90 (34) | |
| Nurse/physician co‐chair | 31 (12) | |
| Other | 17 (6) | |
| Clinical specialty of chaira | ||
| Pulmonary/critical care | 79 (35) | |
| Emergency medicine | 71 (31) | |
| Anesthesia/critical care | 43 (19) | |
| Cardiology | 38 (17) | |
| Other | 32 (14) | |
| Hospital medicine | 23 (10) | |
| Predetermined cardiac arrest team structure | 334 (77) | |
| Notifications of respondersa | ||
| Hospital‐wide PA system | 406 (93) | |
| Pager/calls to individuals | 230 (53) | |
| Local alarm | 49 (11) | |
| Equipment | ||
| AEDs used as primary defibrillator by location | ||
| High‐acuity inpatient areas | 69 (16) | |
| Low‐acuity inpatient areas | 109 (26) | |
| Outpatient areas | 206 (51) | Class IIb, LOE Cb |
| Public areas | 263 (78) | Class IIb, LOE Cb |
| Defibrillator throughout hospital | ||
| Same brand and model | 281 (66) | |
| Same brand, different models | 93 (22) | |
| Different brands | 54 (13) | |
| CPR assist technology useda | ||
| None | 291 (70) | |
| Capnography | 106 (25) | Class IIb, LOE Cb |
| Mechanical CPR | 25 (6) | Class IIb, LOE B/Cbc |
| Feedback device | 17 (4) | Class IIa, LOE B |
| Quality improvement | ||
| IHCA tracked | 336 (82) | Supportedbd |
| Data reviewed | Supportedbd | |
| Data not tracked/never reviewed | 85 (20) | |
| Intermittently | 53 (12) | |
| Routinely | 287 (68) | |
| Routine cardiac arrest case reviews/debriefing | 149 (34) | Class IIa, LOE C |
| Dedicated staff to resuscitation QI | 196 (49) | |
| Full‐time equivalent staffing, median (IQR) | 0.5 (0.251.2) | |
| Routine simulated resuscitation training | 268 (62) | |
| Pre‐ and postarrest measures | ||
| Hospitals with RRT | 391 (91) | Class I, LOE Cb |
| Formal RRT‐specific training | ||
| Never | 50 (14) | |
| Once | 110 (30) | |
| Recurrent | 163 (45) | |
| TH protocol/order set in place | 252 (58) | |
| Percent of patients with ROSC receiving TH | Class IIb, LOE Bb | |
| <5% | 309 (74) | |
| 5%25% | 68 (16) | |
| 26%50% | 11 (3) | |
| 51%75% | 10 (2) | |
| >75% | 18 (4) | |
Hospitals reported that routine responders to IHCA events included respiratory therapists (n=414 [95%]), critical care nurses (n=406 [93%]), floor nurses (n=396 [90%]), attending physicians (n=392 [89%]), physician trainees (n=162 [37%]), and pharmacists (n=210 [48%]). Figure 1 shows the distribution of responders and team leaders by hospital type. Of the nonteaching hospitals, attending‐level physicians were likely to respond at 94% (265/281) and routinely lead the resuscitations at 84% (236/281), whereas, of major teaching hospitals, attending physicians were only likely to respond at 71% (30/42) and routinely lead at 19% (8/42).
Two‐thirds of the hospitals had a CPR committee (n=270 [66%]), and 196 (49%) had some staff time dedicated to resuscitation quality improvement. Hospitals with a specific committee dedicated to resuscitation and/or dedicated staff for resuscitation quality improvement were more likely to routinely track cardiac arrest data (odds ratio [OR]: 3.64, 95% confidence interval [CI]: 2.056.47 and OR: 2.02, 95% CI: 1.16‐3.54, respectively) and review the data (OR: 2.67, 95% CI: 1.45‐4.92 and OR: 2.18, 95% CI: 1.22‐3.89, respectively), after adjusting for teaching status and hospital size. These hospitals were also more likely to engage in simulation training and debriefing (Table 3).
| CPR Committee, n=406 | Dedicated QI Staff, n=398 | |
|---|---|---|
| ||
| IHCA tracking | 3.64 (2.056.47) | 2.02 (1.16‐3.54) |
| Routinely review | 2.67 (1.45‐4.92) | 2.18 (1.22‐3.89) |
| Simulation training | 2.63 (1.66‐4.18) | 1.89 (1.24‐2.89) |
| Debriefing | 3.19 (1.89‐5.36) | 2.14 (1.39‐3.32) |
Ninety percent (n=391) of respondents agreed that there is room for improvement in resuscitation practice at my hospital, and 70% (n=302) agreed that improved resuscitation would translate into improved patient outcomes. Overall, 78% (n=338) cited at least 1 barrier to improved resuscitation quality, of which the lack of adequate training (n=233 [54%]) and the lack of an appropriate champion (n=230 [53%]) were the most common. In subgroup analysis, nonteaching hospitals were significantly more likely to report the lack of a champion than their teaching counterparts (P=0.001) (Figure 2). In addition, we analyzed the data by hospitals that reported lack of a champion was not a barrier and compared them to those for whom it was, and found significantly higher adherence across all the measures in Table 2 supported by the 2010 guidelines, with the exception of real‐time feedback (data not shown).
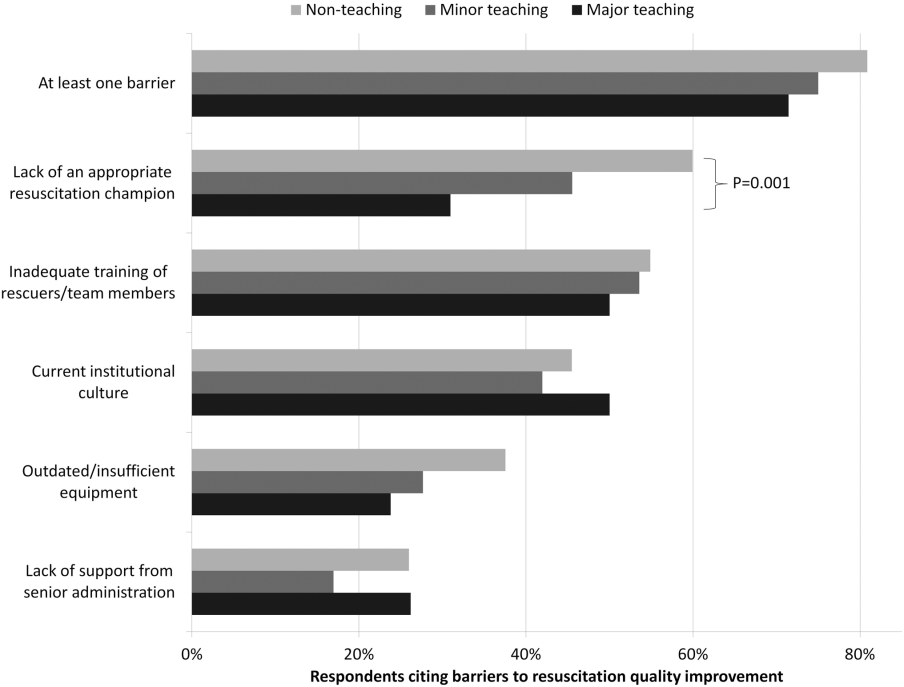
DISCUSSION
In this nationally representative sample of hospitals, we found considerable variability in cardiac arrest and resuscitation structures and processes, suggesting potential areas to target for improvement. Some practices, including use of RRTs and defibrillator standardization, were fairly routine, whereas others, such as therapeutic hypothermia and CPR assist technology, were rarely utilized. Quality initiatives, such as data tracking and review, simulation training, and debriefing were variable.
Several factors likely contribute to the variable implementation of evidence‐based practices. Guidelines alone have been shown to have little impact on practice by physicians in general.[11] This is supported by the lack of correlation we found between the presence, absence or strength of specific American Heart Association (AHA) emergency cardiovascular care treatment recommendations and the percent of hospitals reporting performing that measure. It is possible that other factors, such as a lack of familiarity or agreement with those guidelines, or the presence of external barriers, may be contributing.[12, 13] Specifically, the importance of a clinical champion was supported by our finding that hospitals reporting lack of a champion as a barrier were less likely to be adherent with guidelines. However, because the study did not directly test the impact of a champion, we wanted to be careful to avoid overstating or editorializing our results.
Some of the variability may also be related to the resource intensiveness of the practice. Routine simulation training and debriefing interventions, for example, are time intensive and require trained personnel to institute. That may explain the correlation we noted between these practices and the presence of CPR committee and dedicated personnel. The use of dedicated personnel was rare in this study, with less than half of respondents reporting any dedicated staff and a median of 0.5 full‐time equivalents for those reporting positively. This is in stark contrast to the routine use of resuscitation officers (primarily nurses dedicated to overseeing resuscitation practices and education at the hospital) in the United Kingdom.[14] Such a resuscitation officer model adopted by US hospitals could improve the quality and intensity of resuscitation care approaches.
Particularly surprising was the high rate of respondents (70%) reporting that they do not utilize any CPR assist technology. In the patient who does not have an arterial line, use of quantitative capnography is the best measure of cardiac output during cardiac arrest, yet only one‐quarter of hospitals reported using it, with no discrepancy between hospital type or size. A recent summit of national resuscitation experts expounded on the AHA guidelines suggesting that end‐tidal carbon dioxide should be used in all arrests to guide the quality of CPR with a goal value of >20.[8] Similarly, CPR feedback devices have an even higher level of evidence recommendation in the 2010 AHA guidelines than capnography, yet only 4% of hospitals reported utilizing them. Although it is true that introducing these CPR assist technologies into a hospital would require some effort on the part of hospital leadership, it is important to recognize the potential role such devices might play in the larger context of a resuscitation quality program to optimize clinical outcomes from IHCA.
Several differences were noted between hospitals based on teaching status. Although all hospitals were more likely to rely on physicians to lead resuscitations, nonteaching hospitals were more likely to report routine leadership by nurses and pharmacists. Nonteaching hospitals were also less likely to have a CPR committee, even after adjusting for hospital size. In addition, these hospitals were also more likely to report the lack of a clinical champion as a barrier to quality improvement.
There were several limitations to this study. First, this was a descriptive survey that was not tied to outcomes. As such, we are unable to draw conclusions about which practices correlate with decreased incidence of cardiac arrest and improved survival. Second, this was an optional survey with a somewhat limited response rate. Even though the characteristics of the nonresponding hospitals were similar to the responding hospitals, we cannot rule out the possibility that a selection bias was introduced, which would likely overestimate adherence to the guidelines. Self‐reported responses may have introduced additional errors. Finally, the short interval between the release of the 2010 guidelines and the administration of the first survey may have contributed to the variability in implementation of some practices, but many of the recommendations had been previously included in the 2005 guidelines.
We conclude that there is wide variability between hospitals and within practices for resuscitation care. Future work should seek to understand which practices are associated with improved patient outcomes and how best to implement these practices in a more uniform fashion.
Acknowledgements
The authors thank Nancy Hinckley, who championed the study; David Chearo, Christelle Marpaud, and Martha Van Haitsma of the University of Chicago Survey Lab for their assistance in formulating and distributing the survey; and JoAnne Resnic, Nicole Twu, and Frank Zadravecz for administrative support.
Disclosures: This study was supported by the Society of Hospital Medicine with a grant from Philips Healthcare (Andover, MA). Dr. Edelson is supported by a career development award from the National Heart, Lung, and Blood Institute (K23 HL097157). In addition, she has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). Dr. Hunt has received research support from the Laerdal Foundation for Acute Medicine (Stavanger, Norway), the Hartwell Foundation (Memphis, TN), and the Arthur Vining Davis Foundation (Jacksonville, FL), and honoraria from the Kansas University Endowment (Kansas City, KS), JCCC (Overland Park, KS), and the UVA School of Medicine (Charlottesville, VA) and the European School of Management (Berlin, Germany). Dr. Mancini is supported in part by an Agency for Healthcare Research and Quality grant (R18HS020416). In addition, she has received research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and honoraria from Sotera Wireless, Inc. (San Diego, CA). Dr. Abella has received research support from the National Institutes of Health (NIH), Medtronic Foundation (Minneapolis, MN), and Philips Healthcare (Andover, MA); has volunteered with the American Heart Association; and received honoraria from Heartsine (Belfast, Ireland), Velomedix (Menlo Park, CA), and Stryker (Kalamazoo, MI). Mr. Miller is employed by the Society of Hospital Medicine.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406.
- , , , et al. Racial differences in survival after in‐hospital cardiac arrest. JAMA. 2009;302(11):1195–1201.
- , , , , . Hospital variation in time to defibrillation after in‐hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265–1273.
- , , , et al. Duration of resuscitation efforts and survival after in‐hospital cardiac arrest: an observational study. Lancet. 2012;380(9852):1473–1481.
- , , , . Delayed time to defibrillation after in‐hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17.
- 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care science. Circulation. 2010;122(18 suppl 3):S640–S946.
- , , , et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435.
- American Hospital Association. 2008 AHA annual survey. AHA data viewer: survey instruments. 2012; Available at: http://www.ahadataviewer.com/about/hospital‐database. Accessed October 11, 2013.
- The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 7th ed. Deerfield, IL: AAPOR; 2011.
- , , , , , . Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321(19):1306–1311.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Cardiopulmonary resuscitation standards for clinical practice and training in the UK. Accid Emerg Nurs. 2005;13(3):171–179.
An estimated 200,000 adult patients suffer cardiac arrest in US hospitals each year, of which <20% survive to hospital discharge.[1, 2] Patient survival from in‐hospital cardiac arrest (IHCA), however, varies widely across hospitals, and may be partly attributed to differences in hospital practices.[3, 4, 5] Although there are data to support specific patient‐level practices in the hospital, such as delivery of electrical shock for ventricular fibrillation within 2 minutes of onset of the lethal rhythm,[6] little is known about in‐hospital systems‐level factors. Similar to patient‐level practices, some organizational and systems level practices are supported by international consensus and guideline recommendations.[7, 8] However, the adoption of these practices is poorly understood. As such, we sought to gain a better understanding of current US hospital practices with regard to IHCA and resuscitation with the hopes of identifying potential targets for improvement in quality and outcomes.
METHODS
We conducted a nationally representative mail survey between May 2011 and November 2011, targeting a stratified random sample of 1000 hospitals. We utilized the US Acute‐Care Hospitals (FY2008) database from the American Hospital Association to determine the total population of 3809 community hospitals (ie, nonfederal government, nonpsychiatric, and nonlong‐term care hospitals).[9] This included general medical and surgical, surgical, cancer, heart, orthopedic, and children's hospitals. These hospitals were stratified into tertiles by annual in‐patient days and teaching status (major, minor, nonteaching), from which our sample was randomly selected (Table 1). We identified each hospital's cardiopulmonary resuscitation (CPR) committee (sometimes known as code committee, code blue committee, or cardiac arrest committee) chair or chief medical/quality officer, to whom the paper‐based survey was addressed, with instructions to forward to the most appropriate person if someone other than the recipient. This study was evaluated by the University of Chicago institutional review board and deemed exempt from further review.

Survey
The survey content was developed by the study investigators and iteratively adapted by consensus and beta testing to require approximately 10 minutes to complete. Questions were edited and formatted by the University of Chicago Survey Lab (Chicago, IL) to be more precise and generalizable. Surveys were mailed in May 2011 and resent twice to nonresponders. A $10 incentive was included in the second mailing. When more than 1 response from a hospital was received, the more complete survey was used, or if equally complete, the responses were combined. All printing, mailing, receipt control, and data entry were performed by the University of Chicago Survey Lab, and data entry was double‐keyed to ensure accuracy.
Response rate was calculated based on the American Association for Public Opinion Research standard response rate formula.[10] It was assumed that the portion of nonresponding cases were ineligible at the same rate of cases for which eligibility was determined. A survey was considered complete if at least 75% of individual questions contained a valid response, partially complete if at least 40% but less than 75% of questions contained a valid response, and a nonresponse if less than 40% was completed. Nonresponses were excluded from the analysis.
Statistical Analysis
Analyses were performed using a statistical software application (Stata version 11.0; StataCorp, College Station, TX). Descriptive statistics were calculated and presented as number (%) or median (interquartile range). A [2] statistic was used to assess bias in response rate. We determined a priori 2 indicators of resource allocation (availability of a CPR committee and dedicated personnel for resuscitation quality improvement) and tested their association with quality improvement initiatives, using logistic regression to adjust for hospital teaching status and number of admissions as potential confounders. All tests of significance used a 2‐sided P<0.05.
RESULTS
Responses were received from 439 hospitals (425 complete and 14 partially complete), yielding a response rate of 44%. One subject ID was removed from the survey and could not be identified, so it was excluded from any analyses. Hospital demographics were similar between responders and nonresponders (P=0.50) (Table 1). Respondents who filled out the surveys included chief medical/quality officers (n=143 [33%]), chairs of CPR committees (n=64 [15%]), members of CPR committees (n=29 [7%]), chiefs of staff (n=33 [8%]), resuscitation officers/nurses (n=27 [6%]), chief nursing officers (n=13 [3%]), and others (n=131 [30%]).
| Teaching Status | Annual Inpatient Days | Total | ||
|---|---|---|---|---|
| <17,695 | 17,695‐52,500 | >52,500 | ||
| ||||
| Major | 1/2 (50) | 1/8 (13) | 40/82 (49) | 42/92 (46) |
| Minor | 13/39 (33) | 40/89 (45) | 62/133 (47) | 115/261 (44) |
| Nonteaching | 141/293 (48) | 100/236 (42) | 40/118 (34) | 281/647 (43) |
| Total | 156/335 (47) | 143/335 (43) | 145/336 (43) | 438/1,000 (44) |
Table 2 summarizes structure, equipment, quality improvement, and pre‐ and postarrest practices across the hospitals. Of note, 77% of hospitals (n=334) reported having a predesignated, dedicated code team, and 66% (n=281) reported standardized defibrillator make and model throughout their hospital. However, less than one‐third of hospitals utilized any CPR assist technology (eg, CPR quality sensor or mechanical CPR device). The majority of hospitals reported having a rapid response team (RRT) (n=391 [91%]). Although a therapeutic hypothermia protocol for postarrest care was in place in over half of hospitals (n=252 [58%]), utilization of hypothermia for patients with return of spontaneous circulation was infrequent.
| Value | 2010 AHA Guidelines | |
|---|---|---|
| ||
| Structure | ||
| Existing CPR committee | 270 (66) | |
| CPR chair | ||
| Physician only | 129 (48) | |
| Nurse only | 90 (34) | |
| Nurse/physician co‐chair | 31 (12) | |
| Other | 17 (6) | |
| Clinical specialty of chaira | ||
| Pulmonary/critical care | 79 (35) | |
| Emergency medicine | 71 (31) | |
| Anesthesia/critical care | 43 (19) | |
| Cardiology | 38 (17) | |
| Other | 32 (14) | |
| Hospital medicine | 23 (10) | |
| Predetermined cardiac arrest team structure | 334 (77) | |
| Notifications of respondersa | ||
| Hospital‐wide PA system | 406 (93) | |
| Pager/calls to individuals | 230 (53) | |
| Local alarm | 49 (11) | |
| Equipment | ||
| AEDs used as primary defibrillator by location | ||
| High‐acuity inpatient areas | 69 (16) | |
| Low‐acuity inpatient areas | 109 (26) | |
| Outpatient areas | 206 (51) | Class IIb, LOE Cb |
| Public areas | 263 (78) | Class IIb, LOE Cb |
| Defibrillator throughout hospital | ||
| Same brand and model | 281 (66) | |
| Same brand, different models | 93 (22) | |
| Different brands | 54 (13) | |
| CPR assist technology useda | ||
| None | 291 (70) | |
| Capnography | 106 (25) | Class IIb, LOE Cb |
| Mechanical CPR | 25 (6) | Class IIb, LOE B/Cbc |
| Feedback device | 17 (4) | Class IIa, LOE B |
| Quality improvement | ||
| IHCA tracked | 336 (82) | Supportedbd |
| Data reviewed | Supportedbd | |
| Data not tracked/never reviewed | 85 (20) | |
| Intermittently | 53 (12) | |
| Routinely | 287 (68) | |
| Routine cardiac arrest case reviews/debriefing | 149 (34) | Class IIa, LOE C |
| Dedicated staff to resuscitation QI | 196 (49) | |
| Full‐time equivalent staffing, median (IQR) | 0.5 (0.251.2) | |
| Routine simulated resuscitation training | 268 (62) | |
| Pre‐ and postarrest measures | ||
| Hospitals with RRT | 391 (91) | Class I, LOE Cb |
| Formal RRT‐specific training | ||
| Never | 50 (14) | |
| Once | 110 (30) | |
| Recurrent | 163 (45) | |
| TH protocol/order set in place | 252 (58) | |
| Percent of patients with ROSC receiving TH | Class IIb, LOE Bb | |
| <5% | 309 (74) | |
| 5%25% | 68 (16) | |
| 26%50% | 11 (3) | |
| 51%75% | 10 (2) | |
| >75% | 18 (4) | |
Hospitals reported that routine responders to IHCA events included respiratory therapists (n=414 [95%]), critical care nurses (n=406 [93%]), floor nurses (n=396 [90%]), attending physicians (n=392 [89%]), physician trainees (n=162 [37%]), and pharmacists (n=210 [48%]). Figure 1 shows the distribution of responders and team leaders by hospital type. Of the nonteaching hospitals, attending‐level physicians were likely to respond at 94% (265/281) and routinely lead the resuscitations at 84% (236/281), whereas, of major teaching hospitals, attending physicians were only likely to respond at 71% (30/42) and routinely lead at 19% (8/42).
Two‐thirds of the hospitals had a CPR committee (n=270 [66%]), and 196 (49%) had some staff time dedicated to resuscitation quality improvement. Hospitals with a specific committee dedicated to resuscitation and/or dedicated staff for resuscitation quality improvement were more likely to routinely track cardiac arrest data (odds ratio [OR]: 3.64, 95% confidence interval [CI]: 2.056.47 and OR: 2.02, 95% CI: 1.16‐3.54, respectively) and review the data (OR: 2.67, 95% CI: 1.45‐4.92 and OR: 2.18, 95% CI: 1.22‐3.89, respectively), after adjusting for teaching status and hospital size. These hospitals were also more likely to engage in simulation training and debriefing (Table 3).
| CPR Committee, n=406 | Dedicated QI Staff, n=398 | |
|---|---|---|
| ||
| IHCA tracking | 3.64 (2.056.47) | 2.02 (1.16‐3.54) |
| Routinely review | 2.67 (1.45‐4.92) | 2.18 (1.22‐3.89) |
| Simulation training | 2.63 (1.66‐4.18) | 1.89 (1.24‐2.89) |
| Debriefing | 3.19 (1.89‐5.36) | 2.14 (1.39‐3.32) |
Ninety percent (n=391) of respondents agreed that there is room for improvement in resuscitation practice at my hospital, and 70% (n=302) agreed that improved resuscitation would translate into improved patient outcomes. Overall, 78% (n=338) cited at least 1 barrier to improved resuscitation quality, of which the lack of adequate training (n=233 [54%]) and the lack of an appropriate champion (n=230 [53%]) were the most common. In subgroup analysis, nonteaching hospitals were significantly more likely to report the lack of a champion than their teaching counterparts (P=0.001) (Figure 2). In addition, we analyzed the data by hospitals that reported lack of a champion was not a barrier and compared them to those for whom it was, and found significantly higher adherence across all the measures in Table 2 supported by the 2010 guidelines, with the exception of real‐time feedback (data not shown).

DISCUSSION
In this nationally representative sample of hospitals, we found considerable variability in cardiac arrest and resuscitation structures and processes, suggesting potential areas to target for improvement. Some practices, including use of RRTs and defibrillator standardization, were fairly routine, whereas others, such as therapeutic hypothermia and CPR assist technology, were rarely utilized. Quality initiatives, such as data tracking and review, simulation training, and debriefing were variable.
Several factors likely contribute to the variable implementation of evidence‐based practices. Guidelines alone have been shown to have little impact on practice by physicians in general.[11] This is supported by the lack of correlation we found between the presence, absence or strength of specific American Heart Association (AHA) emergency cardiovascular care treatment recommendations and the percent of hospitals reporting performing that measure. It is possible that other factors, such as a lack of familiarity or agreement with those guidelines, or the presence of external barriers, may be contributing.[12, 13] Specifically, the importance of a clinical champion was supported by our finding that hospitals reporting lack of a champion as a barrier were less likely to be adherent with guidelines. However, because the study did not directly test the impact of a champion, we wanted to be careful to avoid overstating or editorializing our results.
Some of the variability may also be related to the resource intensiveness of the practice. Routine simulation training and debriefing interventions, for example, are time intensive and require trained personnel to institute. That may explain the correlation we noted between these practices and the presence of CPR committee and dedicated personnel. The use of dedicated personnel was rare in this study, with less than half of respondents reporting any dedicated staff and a median of 0.5 full‐time equivalents for those reporting positively. This is in stark contrast to the routine use of resuscitation officers (primarily nurses dedicated to overseeing resuscitation practices and education at the hospital) in the United Kingdom.[14] Such a resuscitation officer model adopted by US hospitals could improve the quality and intensity of resuscitation care approaches.
Particularly surprising was the high rate of respondents (70%) reporting that they do not utilize any CPR assist technology. In the patient who does not have an arterial line, use of quantitative capnography is the best measure of cardiac output during cardiac arrest, yet only one‐quarter of hospitals reported using it, with no discrepancy between hospital type or size. A recent summit of national resuscitation experts expounded on the AHA guidelines suggesting that end‐tidal carbon dioxide should be used in all arrests to guide the quality of CPR with a goal value of >20.[8] Similarly, CPR feedback devices have an even higher level of evidence recommendation in the 2010 AHA guidelines than capnography, yet only 4% of hospitals reported utilizing them. Although it is true that introducing these CPR assist technologies into a hospital would require some effort on the part of hospital leadership, it is important to recognize the potential role such devices might play in the larger context of a resuscitation quality program to optimize clinical outcomes from IHCA.
Several differences were noted between hospitals based on teaching status. Although all hospitals were more likely to rely on physicians to lead resuscitations, nonteaching hospitals were more likely to report routine leadership by nurses and pharmacists. Nonteaching hospitals were also less likely to have a CPR committee, even after adjusting for hospital size. In addition, these hospitals were also more likely to report the lack of a clinical champion as a barrier to quality improvement.
There were several limitations to this study. First, this was a descriptive survey that was not tied to outcomes. As such, we are unable to draw conclusions about which practices correlate with decreased incidence of cardiac arrest and improved survival. Second, this was an optional survey with a somewhat limited response rate. Even though the characteristics of the nonresponding hospitals were similar to the responding hospitals, we cannot rule out the possibility that a selection bias was introduced, which would likely overestimate adherence to the guidelines. Self‐reported responses may have introduced additional errors. Finally, the short interval between the release of the 2010 guidelines and the administration of the first survey may have contributed to the variability in implementation of some practices, but many of the recommendations had been previously included in the 2005 guidelines.
We conclude that there is wide variability between hospitals and within practices for resuscitation care. Future work should seek to understand which practices are associated with improved patient outcomes and how best to implement these practices in a more uniform fashion.
Acknowledgements
The authors thank Nancy Hinckley, who championed the study; David Chearo, Christelle Marpaud, and Martha Van Haitsma of the University of Chicago Survey Lab for their assistance in formulating and distributing the survey; and JoAnne Resnic, Nicole Twu, and Frank Zadravecz for administrative support.
Disclosures: This study was supported by the Society of Hospital Medicine with a grant from Philips Healthcare (Andover, MA). Dr. Edelson is supported by a career development award from the National Heart, Lung, and Blood Institute (K23 HL097157). In addition, she has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). Dr. Hunt has received research support from the Laerdal Foundation for Acute Medicine (Stavanger, Norway), the Hartwell Foundation (Memphis, TN), and the Arthur Vining Davis Foundation (Jacksonville, FL), and honoraria from the Kansas University Endowment (Kansas City, KS), JCCC (Overland Park, KS), and the UVA School of Medicine (Charlottesville, VA) and the European School of Management (Berlin, Germany). Dr. Mancini is supported in part by an Agency for Healthcare Research and Quality grant (R18HS020416). In addition, she has received research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and honoraria from Sotera Wireless, Inc. (San Diego, CA). Dr. Abella has received research support from the National Institutes of Health (NIH), Medtronic Foundation (Minneapolis, MN), and Philips Healthcare (Andover, MA); has volunteered with the American Heart Association; and received honoraria from Heartsine (Belfast, Ireland), Velomedix (Menlo Park, CA), and Stryker (Kalamazoo, MI). Mr. Miller is employed by the Society of Hospital Medicine.
An estimated 200,000 adult patients suffer cardiac arrest in US hospitals each year, of which <20% survive to hospital discharge.[1, 2] Patient survival from in‐hospital cardiac arrest (IHCA), however, varies widely across hospitals, and may be partly attributed to differences in hospital practices.[3, 4, 5] Although there are data to support specific patient‐level practices in the hospital, such as delivery of electrical shock for ventricular fibrillation within 2 minutes of onset of the lethal rhythm,[6] little is known about in‐hospital systems‐level factors. Similar to patient‐level practices, some organizational and systems level practices are supported by international consensus and guideline recommendations.[7, 8] However, the adoption of these practices is poorly understood. As such, we sought to gain a better understanding of current US hospital practices with regard to IHCA and resuscitation with the hopes of identifying potential targets for improvement in quality and outcomes.
METHODS
We conducted a nationally representative mail survey between May 2011 and November 2011, targeting a stratified random sample of 1000 hospitals. We utilized the US Acute‐Care Hospitals (FY2008) database from the American Hospital Association to determine the total population of 3809 community hospitals (ie, nonfederal government, nonpsychiatric, and nonlong‐term care hospitals).[9] This included general medical and surgical, surgical, cancer, heart, orthopedic, and children's hospitals. These hospitals were stratified into tertiles by annual in‐patient days and teaching status (major, minor, nonteaching), from which our sample was randomly selected (Table 1). We identified each hospital's cardiopulmonary resuscitation (CPR) committee (sometimes known as code committee, code blue committee, or cardiac arrest committee) chair or chief medical/quality officer, to whom the paper‐based survey was addressed, with instructions to forward to the most appropriate person if someone other than the recipient. This study was evaluated by the University of Chicago institutional review board and deemed exempt from further review.

Survey
The survey content was developed by the study investigators and iteratively adapted by consensus and beta testing to require approximately 10 minutes to complete. Questions were edited and formatted by the University of Chicago Survey Lab (Chicago, IL) to be more precise and generalizable. Surveys were mailed in May 2011 and resent twice to nonresponders. A $10 incentive was included in the second mailing. When more than 1 response from a hospital was received, the more complete survey was used, or if equally complete, the responses were combined. All printing, mailing, receipt control, and data entry were performed by the University of Chicago Survey Lab, and data entry was double‐keyed to ensure accuracy.
Response rate was calculated based on the American Association for Public Opinion Research standard response rate formula.[10] It was assumed that the portion of nonresponding cases were ineligible at the same rate of cases for which eligibility was determined. A survey was considered complete if at least 75% of individual questions contained a valid response, partially complete if at least 40% but less than 75% of questions contained a valid response, and a nonresponse if less than 40% was completed. Nonresponses were excluded from the analysis.
Statistical Analysis
Analyses were performed using a statistical software application (Stata version 11.0; StataCorp, College Station, TX). Descriptive statistics were calculated and presented as number (%) or median (interquartile range). A [2] statistic was used to assess bias in response rate. We determined a priori 2 indicators of resource allocation (availability of a CPR committee and dedicated personnel for resuscitation quality improvement) and tested their association with quality improvement initiatives, using logistic regression to adjust for hospital teaching status and number of admissions as potential confounders. All tests of significance used a 2‐sided P<0.05.
RESULTS
Responses were received from 439 hospitals (425 complete and 14 partially complete), yielding a response rate of 44%. One subject ID was removed from the survey and could not be identified, so it was excluded from any analyses. Hospital demographics were similar between responders and nonresponders (P=0.50) (Table 1). Respondents who filled out the surveys included chief medical/quality officers (n=143 [33%]), chairs of CPR committees (n=64 [15%]), members of CPR committees (n=29 [7%]), chiefs of staff (n=33 [8%]), resuscitation officers/nurses (n=27 [6%]), chief nursing officers (n=13 [3%]), and others (n=131 [30%]).
| Teaching Status | Annual Inpatient Days | Total | ||
|---|---|---|---|---|
| <17,695 | 17,695‐52,500 | >52,500 | ||
| ||||
| Major | 1/2 (50) | 1/8 (13) | 40/82 (49) | 42/92 (46) |
| Minor | 13/39 (33) | 40/89 (45) | 62/133 (47) | 115/261 (44) |
| Nonteaching | 141/293 (48) | 100/236 (42) | 40/118 (34) | 281/647 (43) |
| Total | 156/335 (47) | 143/335 (43) | 145/336 (43) | 438/1,000 (44) |
Table 2 summarizes structure, equipment, quality improvement, and pre‐ and postarrest practices across the hospitals. Of note, 77% of hospitals (n=334) reported having a predesignated, dedicated code team, and 66% (n=281) reported standardized defibrillator make and model throughout their hospital. However, less than one‐third of hospitals utilized any CPR assist technology (eg, CPR quality sensor or mechanical CPR device). The majority of hospitals reported having a rapid response team (RRT) (n=391 [91%]). Although a therapeutic hypothermia protocol for postarrest care was in place in over half of hospitals (n=252 [58%]), utilization of hypothermia for patients with return of spontaneous circulation was infrequent.
| Value | 2010 AHA Guidelines | |
|---|---|---|
| ||
| Structure | ||
| Existing CPR committee | 270 (66) | |
| CPR chair | ||
| Physician only | 129 (48) | |
| Nurse only | 90 (34) | |
| Nurse/physician co‐chair | 31 (12) | |
| Other | 17 (6) | |
| Clinical specialty of chaira | ||
| Pulmonary/critical care | 79 (35) | |
| Emergency medicine | 71 (31) | |
| Anesthesia/critical care | 43 (19) | |
| Cardiology | 38 (17) | |
| Other | 32 (14) | |
| Hospital medicine | 23 (10) | |
| Predetermined cardiac arrest team structure | 334 (77) | |
| Notifications of respondersa | ||
| Hospital‐wide PA system | 406 (93) | |
| Pager/calls to individuals | 230 (53) | |
| Local alarm | 49 (11) | |
| Equipment | ||
| AEDs used as primary defibrillator by location | ||
| High‐acuity inpatient areas | 69 (16) | |
| Low‐acuity inpatient areas | 109 (26) | |
| Outpatient areas | 206 (51) | Class IIb, LOE Cb |
| Public areas | 263 (78) | Class IIb, LOE Cb |
| Defibrillator throughout hospital | ||
| Same brand and model | 281 (66) | |
| Same brand, different models | 93 (22) | |
| Different brands | 54 (13) | |
| CPR assist technology useda | ||
| None | 291 (70) | |
| Capnography | 106 (25) | Class IIb, LOE Cb |
| Mechanical CPR | 25 (6) | Class IIb, LOE B/Cbc |
| Feedback device | 17 (4) | Class IIa, LOE B |
| Quality improvement | ||
| IHCA tracked | 336 (82) | Supportedbd |
| Data reviewed | Supportedbd | |
| Data not tracked/never reviewed | 85 (20) | |
| Intermittently | 53 (12) | |
| Routinely | 287 (68) | |
| Routine cardiac arrest case reviews/debriefing | 149 (34) | Class IIa, LOE C |
| Dedicated staff to resuscitation QI | 196 (49) | |
| Full‐time equivalent staffing, median (IQR) | 0.5 (0.251.2) | |
| Routine simulated resuscitation training | 268 (62) | |
| Pre‐ and postarrest measures | ||
| Hospitals with RRT | 391 (91) | Class I, LOE Cb |
| Formal RRT‐specific training | ||
| Never | 50 (14) | |
| Once | 110 (30) | |
| Recurrent | 163 (45) | |
| TH protocol/order set in place | 252 (58) | |
| Percent of patients with ROSC receiving TH | Class IIb, LOE Bb | |
| <5% | 309 (74) | |
| 5%25% | 68 (16) | |
| 26%50% | 11 (3) | |
| 51%75% | 10 (2) | |
| >75% | 18 (4) | |
Hospitals reported that routine responders to IHCA events included respiratory therapists (n=414 [95%]), critical care nurses (n=406 [93%]), floor nurses (n=396 [90%]), attending physicians (n=392 [89%]), physician trainees (n=162 [37%]), and pharmacists (n=210 [48%]). Figure 1 shows the distribution of responders and team leaders by hospital type. Of the nonteaching hospitals, attending‐level physicians were likely to respond at 94% (265/281) and routinely lead the resuscitations at 84% (236/281), whereas, of major teaching hospitals, attending physicians were only likely to respond at 71% (30/42) and routinely lead at 19% (8/42).
Two‐thirds of the hospitals had a CPR committee (n=270 [66%]), and 196 (49%) had some staff time dedicated to resuscitation quality improvement. Hospitals with a specific committee dedicated to resuscitation and/or dedicated staff for resuscitation quality improvement were more likely to routinely track cardiac arrest data (odds ratio [OR]: 3.64, 95% confidence interval [CI]: 2.056.47 and OR: 2.02, 95% CI: 1.16‐3.54, respectively) and review the data (OR: 2.67, 95% CI: 1.45‐4.92 and OR: 2.18, 95% CI: 1.22‐3.89, respectively), after adjusting for teaching status and hospital size. These hospitals were also more likely to engage in simulation training and debriefing (Table 3).
| CPR Committee, n=406 | Dedicated QI Staff, n=398 | |
|---|---|---|
| ||
| IHCA tracking | 3.64 (2.056.47) | 2.02 (1.16‐3.54) |
| Routinely review | 2.67 (1.45‐4.92) | 2.18 (1.22‐3.89) |
| Simulation training | 2.63 (1.66‐4.18) | 1.89 (1.24‐2.89) |
| Debriefing | 3.19 (1.89‐5.36) | 2.14 (1.39‐3.32) |
Ninety percent (n=391) of respondents agreed that there is room for improvement in resuscitation practice at my hospital, and 70% (n=302) agreed that improved resuscitation would translate into improved patient outcomes. Overall, 78% (n=338) cited at least 1 barrier to improved resuscitation quality, of which the lack of adequate training (n=233 [54%]) and the lack of an appropriate champion (n=230 [53%]) were the most common. In subgroup analysis, nonteaching hospitals were significantly more likely to report the lack of a champion than their teaching counterparts (P=0.001) (Figure 2). In addition, we analyzed the data by hospitals that reported lack of a champion was not a barrier and compared them to those for whom it was, and found significantly higher adherence across all the measures in Table 2 supported by the 2010 guidelines, with the exception of real‐time feedback (data not shown).

DISCUSSION
In this nationally representative sample of hospitals, we found considerable variability in cardiac arrest and resuscitation structures and processes, suggesting potential areas to target for improvement. Some practices, including use of RRTs and defibrillator standardization, were fairly routine, whereas others, such as therapeutic hypothermia and CPR assist technology, were rarely utilized. Quality initiatives, such as data tracking and review, simulation training, and debriefing were variable.
Several factors likely contribute to the variable implementation of evidence‐based practices. Guidelines alone have been shown to have little impact on practice by physicians in general.[11] This is supported by the lack of correlation we found between the presence, absence or strength of specific American Heart Association (AHA) emergency cardiovascular care treatment recommendations and the percent of hospitals reporting performing that measure. It is possible that other factors, such as a lack of familiarity or agreement with those guidelines, or the presence of external barriers, may be contributing.[12, 13] Specifically, the importance of a clinical champion was supported by our finding that hospitals reporting lack of a champion as a barrier were less likely to be adherent with guidelines. However, because the study did not directly test the impact of a champion, we wanted to be careful to avoid overstating or editorializing our results.
Some of the variability may also be related to the resource intensiveness of the practice. Routine simulation training and debriefing interventions, for example, are time intensive and require trained personnel to institute. That may explain the correlation we noted between these practices and the presence of CPR committee and dedicated personnel. The use of dedicated personnel was rare in this study, with less than half of respondents reporting any dedicated staff and a median of 0.5 full‐time equivalents for those reporting positively. This is in stark contrast to the routine use of resuscitation officers (primarily nurses dedicated to overseeing resuscitation practices and education at the hospital) in the United Kingdom.[14] Such a resuscitation officer model adopted by US hospitals could improve the quality and intensity of resuscitation care approaches.
Particularly surprising was the high rate of respondents (70%) reporting that they do not utilize any CPR assist technology. In the patient who does not have an arterial line, use of quantitative capnography is the best measure of cardiac output during cardiac arrest, yet only one‐quarter of hospitals reported using it, with no discrepancy between hospital type or size. A recent summit of national resuscitation experts expounded on the AHA guidelines suggesting that end‐tidal carbon dioxide should be used in all arrests to guide the quality of CPR with a goal value of >20.[8] Similarly, CPR feedback devices have an even higher level of evidence recommendation in the 2010 AHA guidelines than capnography, yet only 4% of hospitals reported utilizing them. Although it is true that introducing these CPR assist technologies into a hospital would require some effort on the part of hospital leadership, it is important to recognize the potential role such devices might play in the larger context of a resuscitation quality program to optimize clinical outcomes from IHCA.
Several differences were noted between hospitals based on teaching status. Although all hospitals were more likely to rely on physicians to lead resuscitations, nonteaching hospitals were more likely to report routine leadership by nurses and pharmacists. Nonteaching hospitals were also less likely to have a CPR committee, even after adjusting for hospital size. In addition, these hospitals were also more likely to report the lack of a clinical champion as a barrier to quality improvement.
There were several limitations to this study. First, this was a descriptive survey that was not tied to outcomes. As such, we are unable to draw conclusions about which practices correlate with decreased incidence of cardiac arrest and improved survival. Second, this was an optional survey with a somewhat limited response rate. Even though the characteristics of the nonresponding hospitals were similar to the responding hospitals, we cannot rule out the possibility that a selection bias was introduced, which would likely overestimate adherence to the guidelines. Self‐reported responses may have introduced additional errors. Finally, the short interval between the release of the 2010 guidelines and the administration of the first survey may have contributed to the variability in implementation of some practices, but many of the recommendations had been previously included in the 2005 guidelines.
We conclude that there is wide variability between hospitals and within practices for resuscitation care. Future work should seek to understand which practices are associated with improved patient outcomes and how best to implement these practices in a more uniform fashion.
Acknowledgements
The authors thank Nancy Hinckley, who championed the study; David Chearo, Christelle Marpaud, and Martha Van Haitsma of the University of Chicago Survey Lab for their assistance in formulating and distributing the survey; and JoAnne Resnic, Nicole Twu, and Frank Zadravecz for administrative support.
Disclosures: This study was supported by the Society of Hospital Medicine with a grant from Philips Healthcare (Andover, MA). Dr. Edelson is supported by a career development award from the National Heart, Lung, and Blood Institute (K23 HL097157). In addition, she has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). Dr. Hunt has received research support from the Laerdal Foundation for Acute Medicine (Stavanger, Norway), the Hartwell Foundation (Memphis, TN), and the Arthur Vining Davis Foundation (Jacksonville, FL), and honoraria from the Kansas University Endowment (Kansas City, KS), JCCC (Overland Park, KS), and the UVA School of Medicine (Charlottesville, VA) and the European School of Management (Berlin, Germany). Dr. Mancini is supported in part by an Agency for Healthcare Research and Quality grant (R18HS020416). In addition, she has received research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and honoraria from Sotera Wireless, Inc. (San Diego, CA). Dr. Abella has received research support from the National Institutes of Health (NIH), Medtronic Foundation (Minneapolis, MN), and Philips Healthcare (Andover, MA); has volunteered with the American Heart Association; and received honoraria from Heartsine (Belfast, Ireland), Velomedix (Menlo Park, CA), and Stryker (Kalamazoo, MI). Mr. Miller is employed by the Society of Hospital Medicine.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406.
- , , , et al. Racial differences in survival after in‐hospital cardiac arrest. JAMA. 2009;302(11):1195–1201.
- , , , , . Hospital variation in time to defibrillation after in‐hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265–1273.
- , , , et al. Duration of resuscitation efforts and survival after in‐hospital cardiac arrest: an observational study. Lancet. 2012;380(9852):1473–1481.
- , , , . Delayed time to defibrillation after in‐hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17.
- 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care science. Circulation. 2010;122(18 suppl 3):S640–S946.
- , , , et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435.
- American Hospital Association. 2008 AHA annual survey. AHA data viewer: survey instruments. 2012; Available at: http://www.ahadataviewer.com/about/hospital‐database. Accessed October 11, 2013.
- The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 7th ed. Deerfield, IL: AAPOR; 2011.
- , , , , , . Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321(19):1306–1311.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Cardiopulmonary resuscitation standards for clinical practice and training in the UK. Accid Emerg Nurs. 2005;13(3):171–179.
- , , , , , . Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–2406.
- , , , et al. Racial differences in survival after in‐hospital cardiac arrest. JAMA. 2009;302(11):1195–1201.
- , , , , . Hospital variation in time to defibrillation after in‐hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265–1273.
- , , , et al. Duration of resuscitation efforts and survival after in‐hospital cardiac arrest: an observational study. Lancet. 2012;380(9852):1473–1481.
- , , , . Delayed time to defibrillation after in‐hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17.
- 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care science. Circulation. 2010;122(18 suppl 3):S640–S946.
- , , , et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–435.
- American Hospital Association. 2008 AHA annual survey. AHA data viewer: survey instruments. 2012; Available at: http://www.ahadataviewer.com/about/hospital‐database. Accessed October 11, 2013.
- The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 7th ed. Deerfield, IL: AAPOR; 2011.
- , , , , , . Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321(19):1306–1311.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Cardiopulmonary resuscitation standards for clinical practice and training in the UK. Accid Emerg Nurs. 2005;13(3):171–179.
© 2014 Society of Hospital Medicine