User login
Prescriber adherence to antiemetic guidelines with the new agent trifluridine-tipiracil
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
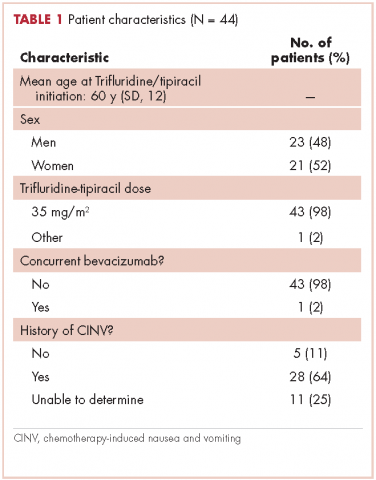
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
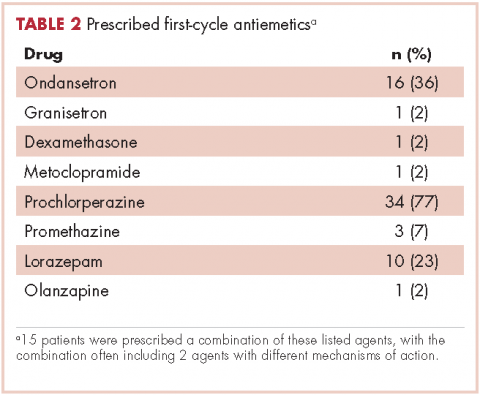
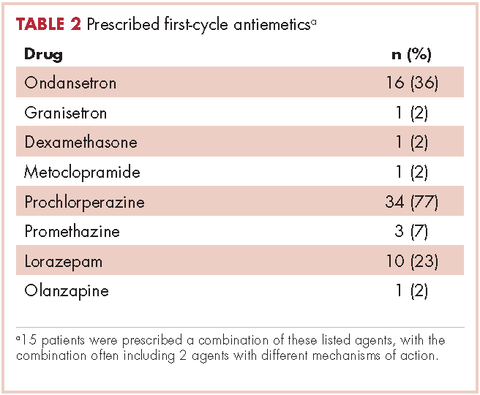
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
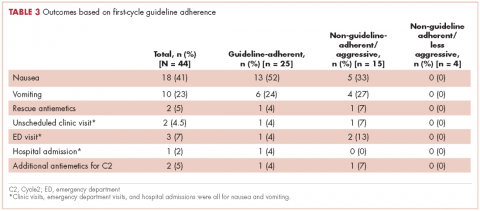
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.