User login
Lemborexant for insomnia
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
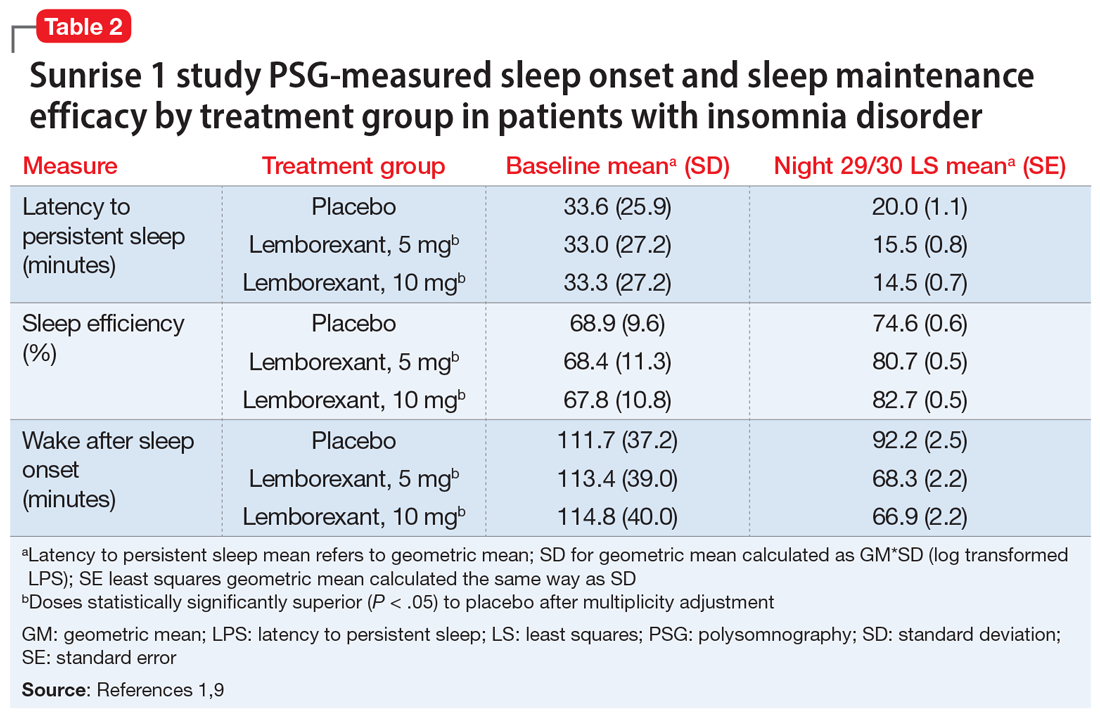
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
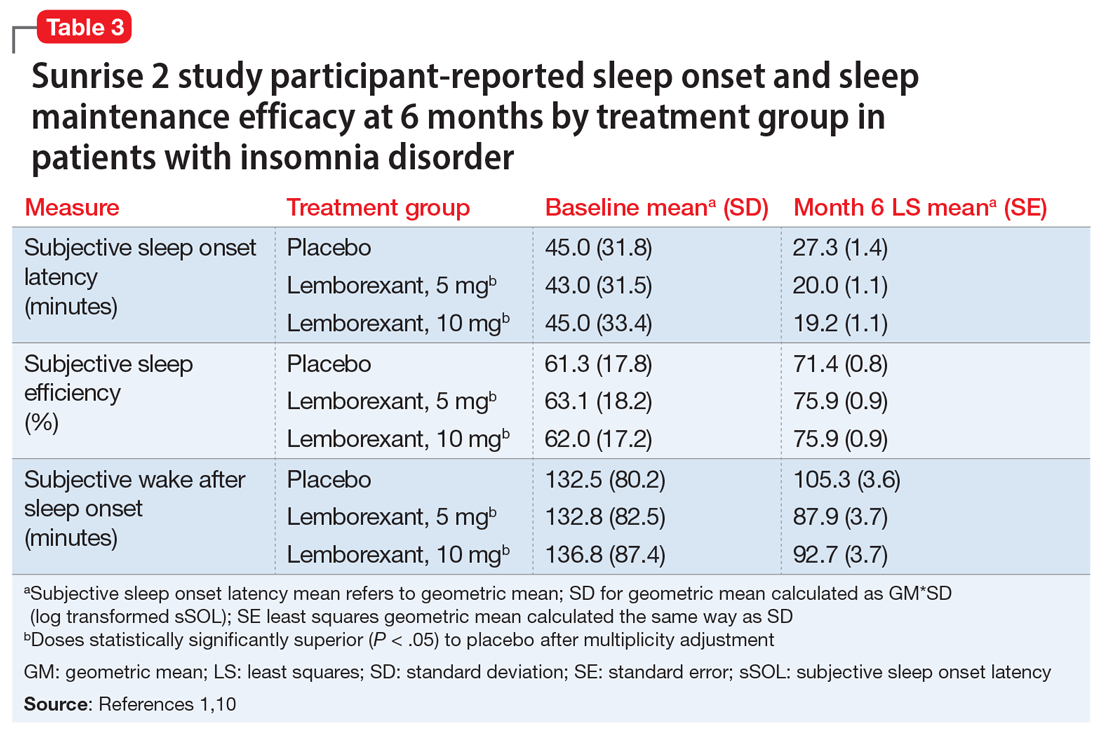
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Suvorexant for sleep-onset insomnia or sleep-maintenance insomnia, or both
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
Suvorexant, FDA-approved to treat insomnia, has demonstrated efficacy in helping patients with insomnia improve their ability to fall asleep and remain asleep (Table 1).1 This first-in-class compound represents a novel mechanism of action to promoting sleep that may avoid some problems associated with other hypnotics.2
Clinical implications
Insomnia is among the most common clinical complaints in psychiatry and medicine. The FDA-approved insomnia medications include several benzodiazepine-receptor agonists (zolpidem, eszopiclone, zaleplon), a melatonin-receptor agonist (ramelteon), and a histamine-receptor antagonist (low-dose doxepin). Suvorexant joins these drugs and is an entirely novel compound that is the first orexin- (also called hypocretin) receptor antagonist approved by the FDA for any indication.
Through a highly targeted mechanism of action, suvorexant could enhance sleep for patients with insomnia, while maintaining an acceptable safety profile.3 The drug should help patients with chronic insomnia, particularly those who have difficulty maintaining sleep—the sleep disturbance pattern that is most challenging to treat pharmacotherapeutically.
Because orexin antagonists have not been used outside of clinical trials, it is too soon to tell whether suvorexant will have the ideal real-world efficacy and safety profile to make it a first-line treatment for insomnia patients, or if it will be reserved for those who have failed a trial of several other treatments.4
In theory, the orexin antagonist approach to treating insomnia could represent a major advance that modulates the fundamental pathology of the disorder.5 The syndrome of chronic insomnia encompasses not just the nighttime sleep disturbance but also an assortment of daytime symptoms that can include fatigue, poor concentration, irritability, and decreased school or work performance but usually not sleepiness. This constellation of nighttime and daytime symptoms could be conceptualized as a manifestation of persistent CNS hyperarousal. Because the orexin system promotes and reinforces arousal, perhaps an orexin antagonist that dampens the level of orexin activity will ameliorate the full spectrum of insomnia symptoms—not simply sedate patients.6
How suvorexant works
Suvorexant is a potent and reversible dual orexin-receptor antagonist. The orexin system, first described in 1998, has a key role in promoting and stabilizing wakefulness.7 Evidence suggests that people with chronic insomnia exhibit a central hyperarousal that perpetuates their sleep difficulty. Accordingly, a targeted pharmaceutical approach that reduces orexin activity should facilitate sleep onset and sleep maintenance for these patients. It is well known that the regulation of sleep and wakefulness depends on the interaction of multiple nuclei within the hypothalamus. Orexinergic neurons in the perifornical-lateral hypothalamic region project widely in the CNS and have especially dense connections with wake-promoting cholinergic, serotonergic, noradrenergic, and histaminergic neurons.6
A precursor prepro-orexin peptide is split into 2 orexin neurotransmitters (orexin A and orexin B). These 2 orexins bind with 2 G-protein-coupled receptors (OX1R and OX2R) that have both overlapping and distinct distributions.7 Suvorexant is highly selective and has similar affinity for OX1R and OX2R, functioning as an antagonist for both.8 Fundamentally, suvorexant enhances sleep by dampening the arousing wake drive.
Pharmacokinetics
Suvorexant is available as an immediate-release tablet with pharmacokinetic properties that offer benefits for sleep onset and maintenance.9 Ingestion under fasting conditions results in a median time to maximum concentration (Tmax) of approximately 2 hours, although the Tmax values vary widely from patient to patient (range 30 minutes to 6 hours). Although suvorexant can be taken with food, there is a modest absorption delay after a high-fat meal, resulting in a further Tmax delay of approximately 1.5 hours.
Suvorexant is primarily metabolized through the cytochrome P450 (CYP) 3A pathway, with limited contribution by CYP2C19. There are no active metabolites. The suvorexant blood level and risk of side effects will be higher with concomitant use of CYP3A inhibitors. The drug should not be administered with strong CYP3A inhibitors; the initial dosage should be reduced with moderate CYP3A inhibitors. Concomitant use of strong CYP3A inducers can result in a low suvorexant level and reduced efficacy.
Suvorexant has little effect on other medications, although a person taking digoxin might experience intestinal P-glycoprotein inhibition with a slight rise in the digoxin level. In a patient taking both medications, monitoring of the digoxin level is recommended.
The elimination half-life of suvorexant is approximately 12 hours, with a steady state in approximately 3 days. Because the half-life of suvorexant is moderately long for a sleep-promoting medication, use of the drug might be associated with residual sleepiness the morning after bedtime dosing. The risk for next-morning sleepiness or impairment should be minimized, however, when using the recommended dosages. Elimination is approximately two-thirds through feces and one-third in the urine.
Suvorexant metabolism can be affected by sex and body mass index. Females and obese people have a modestly elevated exposure to suvorexant, as reflected by the area under the curve and maximum concentration (Cmax). These patients might not require dosage adjustments unless they are obese and female, in which case they should take a lower dosage.
Age and race have not been shown to influence suvorexant metabolism to a significant degree. Patients with renal impairment and those with mild or moderate hepatic impairment do not need dosage adjustment. Suvorexant has not been evaluated in patients with severe hepatic impairment.
Efficacy
Suvorexant showed significant evidence of improved sleep onset and sleep maintenance in patients with insomnia in clinical trials. The key efficacy clinical trials with insomnia patients included a phase-IIb dose-finding study,10 2 similar 3-month phase-III studies,11 and one 12-month phase-III safety study that incorporated efficacy outcomes.12 All these trials included subjective sleep measures and all except for the long-term safety study also incorporated polysomnographic assessment. The specific sleep laboratory outcomes were latency to persistent sleep (LPS), wake after the onset of persistent sleep (WASO), total sleep time (TST), and sleep efficiency (SE). Subjective sleep outcomes were time to sleep onset (sTSO), wake after sleep onset (sWASO), and total sleep time (sTST). Other exploratory endpoints also were assessed. These efficacy and safety studies mostly were performed at dosages considerably higher than those approved by the FDA.
The dose-finding (phase-IIb) trial was conducted with non-geriatric (age 18 to 64) patients with insomnia in a randomized, double-blind, crossover design of two 4-week periods with subjects given a nightly placebo or suvorexant (10 mg, 20 mg, 40 mg, or 80 mg).10 Each of the 4 groups included approximately 60 subjects. The 2 co-primary endpoints were SE at Night 1 and the end of Week 4; secondary endpoints were LPS and WASO. Suvorexant was associated with dosage-related improvements in SE and WASO compared with placebo at both time points. Carryover effects from the period-1 active drug group complicated the analysis of LPS.
The phase-III efficacy and safety trials were performed with 40 mg high dosage (HD) and 20 mg low dosage (LD) groups for adults and with 30 mg HD and 15 mg LD groups for geriatric (age ≥65) patients.11 Two similarly designed 3-month randomized, double-blind, placebo-controlled pivotal efficacy studies assessed objective and subjective sleep measures in 4 groups with non-geriatric (HD and LD) and geriatric (HD and LD) insomnia patients.
After baseline assessment, patients took nightly bedtime doses of placebo; suvorexant, 40 mg or 20 mg (non-geriatric individuals); or suvorexant, 30 mg or 15 mg (geriatric individuals). All subjects kept a daily electronic diary and had polysomnographic recordings performed on Night 1, at the end of Month 1, and at the end of Month 3. Both the individual studies and combined analyses (2,030 subjects) showed that, in non-geriatric and geriatric patients, HD suvorexant resulted in significantly greater improvement in key subjective and objective measures throughout the study (Table 2,9 and Table 3,9), with the exception of a single LPS outcome in 1 study, compared with placebo. The LD dosages also demonstrated efficacy, but to a reduced extent.
Subjective sleep outcomes were assessed in a 1-year randomized, placebo-controlled trial with nightly placebo, suvorexant, 40 mg, for non-geriatric, or suvorexant, 30 mg, for geriatric insomnia patients.12 The 1-year phase was completed with 484 subjects. Key efficacy outcomes were sTST and sTSO changes from baseline during the first month of treatment. Compared with placebo, suvorexant dosages demonstrated significantly greater efficacy, improvements that were sustained throughout the year.
Clinical trials found suvorexant to be generally safe and well tolerated.13 However, specific safety concerns led the FDA to approve the medication at dosages lower than those assessed in the phase-III studies.1
Somnolence was the most common adverse event in clinical trials. In the phase- IIb dose-finding study, somnolence was reported in <1% in the placebo group, but was associated with suvorexant in 2% of the 10 mg group, 5% with 20 mg, 12% with 40 mg, and 11% with 80 mg.9 In the phase-III combined analysis of the 3-month studies, somnolence was reported by 3% in the placebo group and 7% of non-geriatric patients taking 20 mg or geriatric patients taking 15 mg. Somnolence was reported in 8% of women and 3% of men taking the 15 mg or 20 mg dosage in these studies. The 1-year study was performed only with higher suvorexant dosages (30 mg and 40 mg), in comparison with placebo. In this long-term trial, somnolence was reported by 13% of subjects taking suvorexant and 3% taking placebo.
Additional safety issues in trials included excessive daytime sleepiness, impaired driving, suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms.9 Occurrences of these events are rare but have been reported more often among patients taking suvorexant than among those taking placebo.
Unique clinical issues
The U.S. Drug Enforcement Agency has categorized suvorexant as a Schedule IV controlled substance. Although there is no evidence of physiological dependence or withdrawal symptoms with suvorexant, studies with recreational substance abusers have shown that the likeability rating is similar to that of zolpidem.13
Contraindication
Suvorexant is contraindicated in patients with narcolepsy.9 The underlying pathology of narcolepsy involves a marked reduction in orexin functioning with corresponding excessive sleepiness and related symptoms, such as cataplexy, hypnagogic hallucinations, and sleep paralysis. Although suvorexant has not been evaluated in patients with narcolepsy, the drug might, hypothetically, put patients at higher risk of the full spectrum of narcolepsy symptoms.
There are no other contraindications for suvorexant.
Dosing
Suvorexant should be taken no more than once a night within 30 minutes of bedtime and with at least 7 hours before the planned wake time.9 The recommended starting dosage is 10 mg. If this dosage is well tolerated but insufficiently effective, the dosage can be increased to a maximum of 20 mg. The 5-mg dosage is recommended for individuals taking a moderate CYP3A inhibitor. Generally, patients should take the lowest effective dosage.
There are no specified limitations on the duration of suvorexant use. There is no evidence of withdrawal effects when discontinuing the medication. Patients taking suvorexant should be educated about possible next-day effects that might impair driving or other activities that require full mental alertness, especially if they are taking the 20-mg dosage.
Bottom Line
Suvorexant is FDA-approved for treating sleep onset and sleep maintenance insomnia. The drug is a dual orexin-receptor antagonist, which targets persistent CNS hyperarousal. In clinical trials, suvorexant improved the ability to fall asleep and remain asleep in patients with insomnia. It is generally safe and well tolerated. However, these studies evaluated dosages higher than those approved by the FDA.
Related Resources
• Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014; 7(6):711-730.
• Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26(2): 214-224.
Drug Brand Names
Doxepin • Silenor Suvorexant • Belsomra
Digoxin • Lanoxin Zaleplon • Sonata
Eszopiclone • Lunesta Zolpidem • Ambien,
Ramelteon • Rozerem Edluar, Intermezzo
Disclosure
Dr. Neubauer is a consultant to Ferring Pharmaceuticals and Vanda Pharmaceuticals.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.
1. U.S. Food and Drug Administration. Survorexant (orexin receptor antagonist). For insomnia characterized by difficulties with sleep onset and/or maintenance. http:// www.fda.gov/downloads/AdvisoryCommittees/ CommitteesMeetingMaterials/Drugs/Peripheraland CentralNervousSystemDrugsAdvisoryCommittee/ UCM352969.pdf. Published May 22, 2013. Accessed November 24, 2014.
2. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
3. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16(11):1785-1797.
4. Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429-1441.
5. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
6. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
7. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573-585.
8. Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283-293.
9. Belsomra [package insert]. Whitehouse Station, NJ: Merck; 2014.
10. Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265-2274.
11. Ivgy-May N, Snavely D, Minigh J, et al. Efficacy of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated results from 2 similarly designed phase 3 trials. Sleep. 2013;36(abstract supplement): A192.
12. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471.
13. Merck Sharp and Dohme Corporation. Suvorexant advisory committee meeting briefing document. http:// www.fda.govdownloadsadvisorycommittees/committee smeetingmaterials/drugsperipheralandcentralnervous systemdrugsadvisorycommittee/ucm352970.pdf. Published May 22, 2013. Accessed November 24, 2014.
Treatment-resistant insomnia? Ask yourself 8 questions
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
Although many patients with insomnia respond to standard treatments, some continue to experience insufficient sleep. When your patient appears “treatment-resistant,” you may be tempted to add another therapy or try an unorthodox medication. But choosing an appropriate next treatment is impossible without first looking back for a rationale:
- Have you overlooked one of insomnia’s many causes?
- Have you customized treatment for this patient?
- Is he or she unaware of behaviors that may be undermining attempts to sleep?
Refreshing sleep may elude some thoroughly evaluated and optimally treated patients, but they comprise a small minority. You can help most chronic insomnia sufferers by re-evaluating their behaviors, comorbidities, sleep-wake cycles, and medications (Table 1).
Table 1
Recommended approach to treatment-resistant insomnia
| Evaluation |
| Review your patient’s 24-hour sleep cycle, sleepiness, and sleeplessness, and note persistent patterns (a sleep log or diary may help) |
| Re-evaluate stimulating or sedating effects of prescribed and over-the-counter medications, caffeine, and alcohol |
Consider:
|
| Monitor insomnia-related daytime symptoms as key outcome measure |
| Treatment |
| Re-address sleep hygiene (Table 2) |
| Consider cognitive behavioral therapy for insomnia |
| Consider an FDA-approved medication for insomnia (Table 3), customized to your patient’s symptoms |
‘3 Ps’ and 8 questions
Thirty percent of adults experience insomnia at least occasionally, and 10% have persistent insomnia. Women, older persons, and patients with chronic medical conditions such as diabetes mellitus and lung disease have higher insomnia rates than the general population.1
An enormous variety of psychological and physiologic processes may influence sleep (Box 1). Multiple factors may contribute to an individual’s inability to achieve sufficient sleep, and the relative significance of these influences can shift over time. Factors that might trigger an insomnia episode are not necessarily those that maintain sleeplessness.
1 Does the patient have realistic goals for falling asleep and remaining asleep?
Patients view insomnia as being unable to sleep when they believe they should be sleeping. To be diagnosed as a disorder, insomnia must have daytime consequences associated with:
- difficulty falling asleep
- difficulty maintaining sleep
- awakening excessively early
- or experiencing nonrestorative sleep.
Recommendation. Determine how the patient defines “having insomnia” (there are no absolute thresholds). Ask how he or she is functioning during the day. Those who complain of imperfect nighttime sleep may admit that treatment has helped with the daytime symptoms that prompted them to seek treatment.
If daytime symptoms have diminished, reassure the patient that treatment apparently is helping. Patients are less likely to focus on perceived nighttime impairment when their distress about daytime functioning has eased.
Also determine if the patient has followed recommended treatment. Cognitive-behavioral therapy (CBT) may increase adherence to behavioral changes, sleep hygiene, and medication schedules.
2 Have I identified and optimally managed comorbidities?
Identifying comorbidities that may contribute to chronic insomnia is particularly important because managing these conditions may alleviate the sleep disturbance. Pain or discomfort caused by a medical condition may undermine sleep quality. Certain cardiovascular, pulmonary, endocrine, neurologic, rheumatologic, and orthopedic disorders are associated with insomnia.
Most patients experiencing exacerbations of mood and anxiety disorders suffer insomnia, and many other psychiatric disorders are associated with sleep disruption.
Diagnostic subtypes recognized by the American Academy of Sleep Medicine may suggest why recommended treatments have not relieved a patient’s symptoms. Insomnia may be:
- due to a mental disorder, medical condition, drug or substance
- adjustment-related (acute insomnia), psychophysiologic, paradoxical, or idiopathic
- related to inadequate sleep hygiene
- a behavioral characteristic of childhood
- organic (due to an unspecified physiologic condition)
- nonorganic, NOS (not due to a substance or known physiologic condition).
NOS: not otherwise specified
Insomnia often accompanies substance abuse and may continue after the patient stops abusing drugs or alcohol. Abused stimulants and sedatives can worsen sleep quality, and discontinuation can cause acute and chronic sleep disruption.
Recommendation. Treat mood and anxiety disorders independently of insomnia. Minimize pain and discomfort from medical conditions. Address substance abuse, and dispel patients’ notion that alcohol is a sleep aid.
Order sleep laboratory testing for patients at risk for sleep apnea, based on their history, physical exam—including obesity, upper airway anatomy, and neck circumference (collar size ≥17 inches)—and informant reports of snoring and breathing patterns.
3 Is the patient taking medications with stimulating effects?
Because insomnia is highly comorbid with mood and anxiety disorders, patients with insomnia often are prescribed antidepressants. Although some are sedating, antidepressants such as selective serotonin reuptake inhibitors are likely to be stimulating.
Recommendation. When insomnia persists, assess the potential effects of prescribed and over-the-counter (OTC) medications. Consider possible pharmacologic effects of aging that can make patients more sensitive to medications.
Also educate patients about the long-acting effects of caffeine and its varied sources, such as energy drinks and OTC products. Some patients will benefit from completely avoiding caffeine, whereas others may do fine restricting coffee to 1 or 2 cups in the morning. A good general practice is to avoid all caffeine after lunchtime.
Predisposing factors. Some personalities may be predisposed to insomnia. Persons who tend to be anxious, depressive, or emotionally reactive may be at increased risk for developing insomnia.
Precipitating factors may include situational crises, schedule changes, substance or medication use, and psychiatric, medical, and sleep disorders. A careful history allows you to consider precipitating events.
Perpetuating factors that may reinforce and maintain chronic insomnia include:
- maladaptive behaviors, such as napping or using alcohol as a sleep aid
- conditioned hyperarousal, whereby insomnia sufferers experience anxiety and tension associated with preparing for and getting into bed. Sleepless time in bed may reinforce the conditioning, contribute to anxiety and tension, and undermine sleep on future nights.
Source: Reference 2
4 Does the patient’s insomnia have a homeostatic component?
Circadian rhythms and a homeostatic sleep drive are temporally linked in regulating the normal routine of nighttime sleep alternating with day and evening wakefulness.5,6 The sleep drive promotes a sleep-to-waking ratio of approximately 1:2 (an average of 8 hours sleep per 24 hours). Adequate sleep, from the homeostatic perspective, could be achieved during any hours of the day or night.
Acute sleep deprivation may result from extended wakefulness—such as staying up all night to study for an exam. Chronic sleep deprivation may occur during successive 24-hour periods with insufficient sleep. Both patterns are associated with increasing subjective sleepiness and ultimately with cognitive impairment.
- is low throughout the daytime
- rises during the evening as bedtime approaches
- plateaus during nighttime sleep hours
- decreases as the normal morning wake time approaches.
Typically, people are more alert in the evening than at any other time in the 24-hour cycle. As bedtime approaches, rising melatonin interacts with SCN melatonin receptors and decreases circadian arousal. Normal sleep onset then can occur rapidly at bedtime, when the homeostatic sleep drive is unopposed.
Nighttime sleep initially is promoted by the homeostatic sleep drive. However, the homeostatic sleep pressure is reversed by sleep and thus decreases as sleep continues during the night. The circadian system promotes minimum stimulation during the latter sleep hours, sustaining total sleep for approximately 8 hours.
Consequences. Individual circadian timing tendencies may affect when people experience alertness and sleepiness and may be associated with persistent complaints of sleep onset difficulty or early morning awakening.7 Napping may reduce the homeostatic sleepiness available to aid bedtime sleep onset. Mismatched homeostatic and circadian processes often prevent shift workers from achieving satisfactory sleep.
Recommendation. Have the patient keep a sleep log to identify the time and duration of sleep episodes throughout the 24-hour cycle. Actigraphy may provide useful information about sleep-wake patterns.
5 Are circadian rhythm patterns contributing to insomnia?
Overlooking circadian rhythms’ effects on insomnia can lead to apparent treatment failure.8 Although the circadian system typically promotes sleep from about 10 pm to midnight until about 6 to 8 am, some individuals have long-standing predispositions for earlier or later sleep episodes.
An advanced circadian phase leads to sleepiness and the ability to fall asleep early in the evening, followed by a tendency to awaken spontaneously relatively early in the morning. In extreme cases, patients with these “lark” tendencies may be diagnosed with advanced sleep phase disorder. Persistent early morning awakening insomnia and sleep maintenance complaints are common.
A delayed circadian phase is associated with inability to fall asleep at a typical late evening bedtime and difficulty awakening at a desired time the following morning. In extreme cases, individuals may sleep from very late at night until the following afternoon. These markedly delayed schedules may be obvious, but the circadian contribution may not be recognized in less severe cases.
People with this predisposition may achieve optimum sleep by following their delayed circadian tendency, but school and work demands often conflict with this approach. They may develop chronic sleep deprivation from late sleep onset coupled with forced morning awakenings. Complaints of chronic difficulty with sleep onset are common.
Recommendation. Have the patient keep a sleep log to demonstrate advanced or delayed circadian phase tendencies. Determine if the patient is a shift worker who is attempting to sleep in the daytime. Consider prescribing ramelteon—a melatonin agonist—and providing strategic bright light exposure:
- in the evening for advanced circadian phase patients
- in the morning for delayed circadian phase patients.8
6 Is the patient following appropriate sleep hygiene?
Sleep hygiene will not necessarily cure chronic insomnia, but inattention to basic guidelines (Table 2) can undermine other treatments. When re-evaluating patients with chronic insomnia, give special attention to their alcohol and caffeine intake, regularity of bedtime and wake-up times, meal times, and the bedroom environment. Advise patients to remove televisions from the bedroom, for example.
CBT that is effective for chronic insomnia typically blends sleep hygiene with education, cognitive psychotherapy, and specific instructions regarding bedtime schedules.9,10 Relaxation techniques also may be beneficial.
Consider consulting with a sleep specialist if the patient has not been evaluated at a sleep center. Some sleep centers offer CBT.
Table 2
Patient education: Sleep hygiene guidelines
| Try to maintain a regular sleep–wake schedule |
| Avoid afternoon or evening napping |
| Allow yourself enough time in bed for adequate sleep duration (such as 11 PM to 7 AM) |
| Develop a relaxing evening routine for the hours before bedtime |
| Spend some idle time reflecting on the day’s events before going to bed; make a list of concerns and how some might be resolved |
| Reserve the bed for sleep and sex; do not do homework, pay bills, watch TV, or engage in serious domestic discussions in bed |
| Avoid alcohol in the evening |
| Avoid caffeine in the afternoon and evening |
| Minimize annoying noise, light, or temperature extremes |
| Consider a light snack before bedtime |
| Exercise regularly, but not late in the evening |
| Do not try harder and harder to fall asleep; if you can’t sleep, get out of bed and do something else, in another room if possible |
| Avoid smoking |
7 Does the patient regularly experience anxiety and tension as bedtime approaches or spend excessive wakeful time in bed?
Patients who tend to be anxious, depressive, or emotionally reactive are at increased risk for developing an insomnia episode. They then may develop conditioned hyperarousal associated with preparing for and getting into bed, which perpetuates insomnia.
Some patients spend long periods in bed, hoping to achieve any possible sleep that night. Extended time in bed can perpetuate insomnia by increasing frustrating time awake, thereby reinforcing the association between the bed and wakefulness.
Recommendation. CBT often helps ease these conditioned responses.
Stimulus control can help anxious individuals reassociate the bed, bedroom, and bedtime routines with sleep onset, rather than sleep-destructive tension. Advise patients to go to bed in the evening when they feel they can fall asleep. If they do not fall asleep within 10 to 15 minutes or experience their usual worry and frustration about not sleeping, instruct them to leave the bed and try again later. Also tell them to avoid daytime napping.
Sleep restriction therapy may help patients with excessive wakefulness in bed by limiting sleep opportunity to defined hours of the night. For example, a patient who reports getting 5 hours of sleep would be scheduled for 5 hours in bed. If his typical arising time is 7 am, he would not go to bed until 2 am. When his sleep log shows he has slept 90% of the time in bed for 5 consecutive nights, he can go to bed 15 to 30 minutes earlier. Over time, as this process is repeated, patients spend greater amounts of time sleeping while in bed.
Sleep restriction creates a degree of sleep deprivation that may enhance sleep onset and maintenance. Caution patients not to drive or perform hazardous activities while sleep-deprived.
8 Has the patient been prescribed appropriate doses of medications with appropriate indications?
Chronic insomnia sufferers often try to get more sleep by using alcohol, food supplement remedies, and OTC antihistamine sleep aids—none of which has demonstrated efficacy for treating insomnia. Although sedating prescription medications may be recommended for comorbid conditions, many also are prescribed off-label to promote sleep.
Examples include sedating antidepressants, antipsychotics, antihistamines, anticonvulsants, and benzodiazepines that are not indicated for insomnia. Little or no evidence supports these medications as safe and efficacious for treating insomnia, and important safety concerns are associated with their use.
The BZRA category includes 5 benzodiazepines and 4 nonbenzodiazepine formulations. Half-lives vary from approximately 1 hour to several days. Compared with benzodiazepines, nonbenzodiazepines have greater selectivity for GABAA receptor complexes incorporating the alpha-1 sub-unit subtype, which may confer some safety and tolerability advantages. One extended-release formulation is available. All may be beneficial for sleep onset, and some have indications for sleep maintenance difficulty.
Ramelteon is a nonsedating selective melatonin receptor agonist approved for treating insomnia characterized by sleep onset difficulty. This agent—which attenuates evening circadian arousal—may help promote sleep onset and enhance sleep during the early part of the night.
Administration. Inadequate dosing of insomnia medications may cause treatment to fail, but prescribing beyond approved ranges is rarely necessary. High sedative doses increase the risk of adverse effects, and patients may sleep no better. Adverse effects may include somnolence, headache, dizziness, nausea, diarrhea, and anterograde amnesia. Rarely patients may exhibit sleep walking or confused behaviors within a few hours after taking a hypnotic dose.
Recommendation. Customize your selection of FDA-approved insomnia medications. Consider whether your patient needs medication for sleep onset or sleep maintenance. In most cases, prescribe within dosing ranges listed in Table 3
Table 3
Insomnia treatment: FDA-approved medications
| Medication | Recommended dosage (mg) | Elimination half-life (hr) |
|---|---|---|
| Benzodiazepine receptor agonists | ||
| Immediate-release benzodiazepines | ||
| Estazolam | 1 to 2 | 8 to 24 |
| Flurazepam | 15 to 30 | 48 to 120 |
| Quazepam | 7.5 to 15 | 48 to 120 |
| Temazepam | 7.5 to 30 | 8 to 20 |
| Triazolam | 0.125 to 0.25 | 2 to 4 |
| Immediate-release nonbenzodiazepines | ||
| Eszopiclone | 1 to 3 | 5 to 7 |
| Zaleplon | 5 to 20 | 1 |
| Zolpidem | 5 to 10 | 1.5 to 2.4 |
| Extended-release nonbenzodiazepine | ||
| Zolpidem ER | 6.25 to 12.5 | 2.8 to 2.9 |
| Selective melatonin receptor agonist | ||
| Ramelteon | 8 | 1 to 2.6 |
Related Resources
- American Academy of Sleep Medicine. www.aasmnet.org.
- National Sleep Foundation. www.sleepfoundation.org.
- NIH National Center for Sleep Disorders Research. www.nhlbi.nih.gov/about/ncsdr.
Drug brand names
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Triazolam • Halcion
- Zaleplon • Sonata
- Zolpidem • Ambien
- Zolpidem ER • Ambien CR
Disclosure
Dr. Neubauer is a consultant to Neurocrine Biosciences, sanofi-aventis, and Takeda Pharmaceuticals North America and a speaker for sanofi-aventis and Takeda Pharmaceuticals North America.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.
1. National Institutes of Health. State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep 2005;28:1049-57.
2. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53.
3. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
4. International Classification of Sleep Disorders: Diagnostic & Coding Manual, ICSD-2, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
5. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 1999;14:557-68.
6. Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry 2005;66:3-9.
7. Manthena P, Zee PC. Neurobiology of circadian rhythm sleep disorders. Curr Neurol Neurosci Rep 2006;6:163-8.
8. Zee PC, Manthena P. The brain’s master circadian clock: implications and opportunities for therapy of sleep disorders. Sleep Med Rev 2007;11(1):59-70.
9. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172-80.
10. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29:1398-414.