User login
The Implications of Power Mobility on Body Weight in a Veteran Population
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
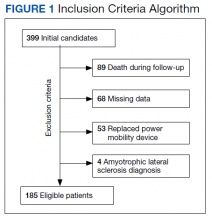
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
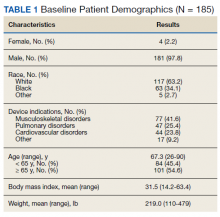
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
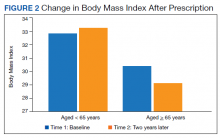
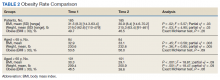
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
The Veterans Health Administration (VHA) clinical practice recommendations endorse a power mobility device (PMD) for individuals with adequate judgment, cognitive ability, and vision who are unable to propel a manual wheelchair or walk community distances despite standard medical and rehabilitative interventions.1 VHA supports the use of a PMD in order to access medical care and accomplish activities of daily living, both at home and in the community for veterans with mobility limitations secondary to cardiovascular disease, neurologic disorders, pulmonary disease, or musculoskeletal disorders. The goal of a PMD use is increased participation in community and social life, improved health maintenance via enhanced access to medical facilities, and an overall enhanced quality of life. However, there is a common concern among health care providers that prescribing a PMD may decrease physical activity, in turn, leading to obesity and increasing morbidity. 2
The prevalence of obesity is increasing in the United States. In the past decade 35.0% of men and 36.8% of women were classified as obese (body mass index [BMI], ≥ 30).3 Recent figures from the Centers for Disease Control and Prevention estimate that the overall prevalence of obesity in Americans is closer to 42.4%.4 The veteran population is not immune to this; a 2014 study of nearly 5 million veterans reported that the prevalence of obesity in this population was 41%.5,6 In addition to obesity being implicated in exacerbating many medical problems, such as osteoarthritis, insulin resistance, and heart disease, obesity also is associated with a significant decrease in lifespan.7 Almost half of adults who report ambulatory dysfunction are obese.8 Given the increased morbidity and mortality as a result of obesity, interventions that may promote weight gain need to be appropriately identified and minimized.
In a retrospective study of 89 veterans, Yang and colleagues demonstrated no significant weight change 1 year after initial PMD prescription.2 Another study of 102 patients noted no significant weight changes 1 year after PMD prescription.9 This study analyzes the effect of PMD prescriptions over a 2-year period on BMI and body weight in a larger population of veterans both as a whole and in BMI/age subgroups.
Methods
The institutional review board at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia, reviewed and approved this study. A waiver of participant consent was approved due to the nature of the research (medical records of patients, some of whom were deceased) and the type of data collected (retrospective data). In addition, each individual was assigned a sequential code to de-identify any personal information. Prosthetics department medical records of consecutive veterans who received PMDs for the first time between January 1, 2011 and June 30, 2012, were reviewed.
Data extracted from the electronic health record (EHR) included demographics, indication for power mobility, weight at time of PMD prescription, weight at 2-years postprescription, and height. Weight readings were considered valid if weight was taken within 3 months of initial prescription and then again within 3 months at the 2-year interval. Individuals without weights recorded in these time frames were excluded. In addition, we excluded medical conditions that might significantly affect body weight, including amyotrophic lateral sclerosis (ALS), amputation during the study period, or history of weight loss surgery. Cancer diagnoses were excluded as they were not an indication for power mobility in the VHA. ALS, though variable in its disease course, was specifically excluded given the likelihood of these patients dying of the natural progression of the disease before the 2-year follow-up period: Median survival times in patients diagnosed with ALS aged > 60 years was < 15 months. 10-12
The EHRs of 399 individuals who received a PMD during the period were reviewed, and 185 veterans met criteria for data analysis. Subject exclusions in the weight and BMI analysis included death during the follow-up period (89), missing data (68), prior PMD users who came in for replacements (53), and ALS (4) (Figure 1). Patients were not excluded based on the presence or absence of intentional weight loss efforts as this information was not readily available through chart review.
Statistical Analysis
The primary outcome measure was the change in BMI and body weight from time 1 (date of PMD prescription) to time 2 (2 years later). Analyses were performed using IBM SPSS Statistics, Version 21. BMI was calculated using the weight (lb) x 703/ (height [inches]).2 Dichotomization of BMI was performed using the conventional cut scores: < 30.0, not obese; and ≥ 30.0, obese. Paired t tests and SPSS general linear model (repeated measures) were used to examine change of BMI from time 1 to time 2. The exact McNemar test was used to examine change in obesity classification across time 1 and time 2. Correlating with Yang’s retrospective observational study, data were analyzed separately for aged < 65 years and aged≥ 65 years.2
Results
Of the 185 veterans, 181 were male (98%); mean age was 67.3 years (range, 26-90); and 55% were aged ≥ 65 years. Musculoskeletal disorders (41.6%) were the most common primary indication for a PMD, followed by pulmonary disorders (25.4%) and cardiovascular disorders (23.8%) (Table 1).
There was a significant decrease in BMI in the first 2 years after receiving a PMD prescription for the first time (estimated marginal means: 31.5 to 30.9 , P = .02). However, age moderated the relationship between BMI and time F[1, 183] = 12.14, P = .001, partial η2 = .06 (Table 2). The 101 subjects aged > 65 years experienced a significant decrease in BMI (estimated marginal means: 30.3 to 29.1, P < .001), whereas the 84 patients aged < 65 years experienced a slight and nonsignificant increase in BMI (estimated marginal means: 32.9 to 33.1, P = .45). BMI was significantly higher for subjects aged < 65 years at Time 1 (F[1, 183] = 4.32, P = .04, partial η2 = .02) and at Time 2 (F[1, 183] = 11.04, P = .001, partial η2 = .06).
Similarly, there was a significant decrease in weight in the first year after receiving a PMD prescription with a change in mean weight from 219.0 to 215.3 lb (P = .3). Again, age moderated the relationship between weight and time (F = 12.81; P < .001; partial η2 = .07). Individuals aged ≥ 65 years experienced a significant decrease in weight (estimated marginal means = 209.4 to 200.9; P < .001), whereas those aged < 65 years experienced a slight and nonsignificant increase in weight (230.6 to 232.6; P = .36). Weight was significantly higher for individuals aged < 65 years at time 1 (F = 5.34; P = .02; partial η2 = .03) and at time 2 (F = 12.18; P = .001; partial η2 = .06).
The percentage of those who were obese (BMI ≥ 30) at time 1 (49.7%) did not significantly change at time 2 (46.5%) (exact McNemar test, P = .26). Similarly, there was no significant change in obesity from time 1 to time 2 for those aged < 65 years (exact McNemar test P = .69) or for those aged ≥ 65 years (exact McNemar test P = .06). Obesity at time 2 was significantly more common in those aged < 65 years (56.0%) than those aged ≥ 65 years (38.6%), χ2 [1] = 5.54; P = .02. Obesity at time 1 did not differ between those aged < 65 years (53.6%) and aged ≥ 65 years (46.5%), η2 [1] = 0.9; P = .34. Obesity moderated the relationship between weight and time (F = 5.10; P = .03; partial η2= .03) in that obese individuals experienced a significant decrease in weight with estimated marginal means (SE) = 264.5 (4.51) to 257.4 (4.97); F = 11.32; P < .001; partial η2 = .06), whereas nonobese individuals had no weight change with estimated marginal means (SE) = 174.0 (4.48) to 173.61 (4.94); F = .03; P < .86; partial η2< .01).
Discussion
This study demonstrated a significant decrease in both weight and BMI at 2 years after the initiation of a PMD in patients aged < 65 years. No significant change was found for obesity rates. However, veterans who met criteria for obesity at the time of PMD prescription saw a significant decrease in their weight at 2 years compared with those who were nonobese.
VHA supports power mobility when there is a clear functional need that cannot be met by rehabilitation, surgical, or medical interventions to enhance veterans’ abilities to access medical care, accomplish necessary tasks of daily living, and to have greater access to their communities. Though limited by strength of association, studies involving PMD users generally found improvement in reported functional outcomes and overall satisfaction with PMD use based on a systematic review.13 Nonetheless, there is an implicit concern among providers that a PMD prescription, by limiting physical activity, may exacerbate obesity trends in potentially high-risk individuals.
However, a controversy exists about whether increasing physical activity alone leads to weight loss. A 2007 study followed 102 sedentary men and 100 women over 1 year randomized to moderately intensive exercise for 60 minutes, 6 days a week vs no intervention.14 The men lost an average of 4 pounds, and women lost an average of 3 pounds after 1 year. The Women’s Health Study divided 39,876 women into high, medium, and low levels of exercise groups. After 10 years, the intense exercise group did not have any significant weight loss.15
Our study was consistent with existing literature in that a PMD prescription did not correlate with weight gain.2,9 In our veteran population aged ≥ 65 years, we observed an opposite trend of weight loss after PMD prescription. Of note, studies have shown that peak body weight occurs in the sixth decade, remains stable until about aged 70 years, and then slowly decreases thereafter, at a rate of 0.1 to 0.2 kg per year.16 This likely explains some of the weight loss trend we observed in our study of veterans aged ≥ 65 years. Possible additional explanations include improved access to health care and to more nutritional foods that promote general health and well-being.
Limitations
The data were gathered from a predominantly male veteran population, potentially limiting generalizability. The health of any individual is determined by the interaction of factors of which body weight is just a single, isolated component. As such, the effect of powered mobility on body weight is not a direct reflection on the effect on overall health. Additionally, there are many factors that may affect an individual’s body weight, such as optimal management of medical comorbidities, which could not be controlled for in this study. Also, while these values can be compared with other veteran populations, this study had no true control group.
Conclusions
Based on the findings of this study with aforementioned limitations, PMD use does not seem to be associated with significant weight changes. Further studies using control groups and assessing comorbidities are needed.
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
1. Perlin J. Clinical practice recommendations for motorized wheeled mobility devices: scooters, pushrim-activated power-assist wheelchairs, power wheelchairs, and power wheelchairs with enhanced function. Published 2004. Accessed August 12, 2021. https://www.prosthetics.va.gov/Docs/Motorized_Wheeled_Mobility_Devices.pdf
2. Yang W, Wilson L, Oda I, Yan J. The effect of providing power mobility on weight change. Am J Phys Med Rehabil. 2007;86(9):746-753. doi:10.1097/PHM.0b013e31813e0645
3. Yang, L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med. 2015; 175(8):1412–1413. doi:10.1001/jamainternmed.2015.2405
4. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2020.
5. Almond N, Kahwati L, Kinsinger L, Porterfield D. The prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173(6):544-549. doi:10.7205/milmed.173.6.544
6. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17. doi:10.1007/s11606-016-3962-1
7. Bray G. Medical consequences of obesity. Int J Clin Endocrinol Metab. 2004;89(6):2583-2589. doi:10.1210/jc.2004-0535
8. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. 2014;25(3):175-185. doi:10.1177/1044207313494236
9. Zagol BW, Krasuski RA. Effect of motorized scooters on quality of life and cardiovascular risk. Am J Cardiol. 2010;105(5):672-676. doi:10.1016/j.amjcard.2009.10.049
10. Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(4):313-320. doi:10.1212/cpj.0b013e3182a1b8ab
11. Wolf J, Safer A, Wöhrle J, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. 2014;14(1):197. doi:10.1186/s12883-014-0197-9
12. Körner S, Hendricks M, Kollewe K, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13:84. doi: 10.1186/1471-2377-13-84
13. Auger CJ, Demers L, Gélinas I, et al. Powered mobility for middle-aged and older adults: systematic review of outcomes and appraisal of published evidence. Am J Phys Med Rehabil. 2008;87(8):666-680. doi:10.1097/PHM.0b013e31816de163
14. McTiernan A, Sorensen B, Irwin M, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15(6):1496-512. doi:10.1038/oby.2007.178
15. Lee IM, Djoussé L, Sesso H, Wang L, Buring JE . Physical activity and weight gain prevention, women’s health study. JAMA. 2010;303(12):1173-1179. doi:10.1001/jama.2010.312
16. Wallace J, Schwartz R. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85(1):15-21. doi:10.1016/s0167-5273(02)00246-2
The VA/DoD Chronic Effects of Neurotrauma Consortium: An Overview at Year 1
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.