User login
Clinical Edge Journal Scan Commentary: Contraception October 2021
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
2021 Update on menopause
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
Among the studies we review in this Update are a follow-up of the US Women’s Health Initiative clinical trials and a large observational study from the United Kingdom, which exlore the impact of different hormone therapies (HTs) on breast cancer risk. We look at the interesting patterns found by authors of a study in Canada that analyzed predictors of unnecessary bilateral salpingo-oophorectomy. In addition, we review a study that investigates whether hormone therapy can be effective, alone or adjunctively, in peri- and postmenopausal women with depression. Finally, Dr. Chrisandra Shufelt and Dr. JoAnn Manson summarize highlights from the recent American Heart Association’s scientific statement on the menopause transition and increasing risk factors for cardiovascular disease, and how this period can be viewed as an opportunity to encourage healthy, cardiovascular risk–reducing behaviors.
Studies clarify menopausal HT’s impact on breast cancer risk
Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
For many menopausal women, the most worrisome concern related to the use of HT is that it might increase breast cancer risk. In the summer and fall of 2020, 2 important articles were published that addressed how the use of menopausal HT impacts the risk of breast cancer.
The Women’s Health Initiative (WHI) represents the largest and longest-term randomized trial assessing the health impacts of systemic HT. A 2013 WHI report found that with a median of 13 years’ cumulative follow-up, estrogen-only HT (ET) reduced the risk for breast cancer while estrogen-progestin therapy (EPT) increased the risk.1 In a July 2020 issue of JAMA, WHI investigators analyzed longer-term data (cumulative median follow-up >20 years), which allowed assessment of whether these trends (breast cancer incidence) persisted and if they led to changes in mortality from breast cancer.2
WHI data on breast cancer risk trends in ET vs EPT users
In the ET trial, in which Chlebowski and colleagues studied 10,739 women with prior hysterectomy, 238 versus 296 new cases of breast cancer were diagnosed in women in the ET versus placebo groups, respectively (annualized incidence, 0.30% [ET] vs 0.37% [placebo]; hazard ratio [HR], 0.78; P = .005). ET also was associated with significantly lower mortality from breast cancer: 30 versus 46 deaths (annualized mortality, 0.031% [ET] vs 0.046% [placebo]; HR, 0.60; P = 0.04).
In the EPT trial, which included 16,608 participants with an intact uterus, EPT compared with placebo was associated with significantly elevated risk for incident breast cancer: 584 versus 447 new cases, respectively (annualized incidence, 0.45% [EPT] vs 0.36% [placebo]; HR, 1.28; P<.001). However, mortality from breast cancer was similar in the EPT and placebo groups: 71 and 53 deaths (annualized mortality, 0.045% [EPT] and 0.035% [placebo]; HR, 1.35; P = .11).2
For women with previous hysterectomy who are considering initiating or continuing ET for treatment of bothersome menopausal symptoms, the breast cancer mortality benefit documented in this long-term WHI analysis could, as editorialists point out, “tip the scales” in favor of ET.3 Furthermore, the mortality benefit raises the possibility that ET could be evaluated as a risk-reduction strategy for selected high-risk menopausal women who have undergone hysterectomy. Although tamoxifen and aromatase inhibitors are approved for breast cancer chemoprophylaxis in high-risk menopausal women, these agents have not been found to lower breast cancer mortality.2
UK data analysis and risk for breast cancer in HT users
In an October 2020 issue of BMJ, Vinogradova and colleagues described their analysis of 2 primary care databases in the United Kingdom that in aggregate included roughly 99,000 women with breast cancer diagnosed between 1998 and 2018 (age range, 50–79; mean age at diagnosis, 63; >95% White); these were matched with more than 450,000 women without breast cancer (controls).4 Analyses were adjusted for smoking, body mass index (BMI), ethnicity, and mammography.
In this study, ever-use of EPT was associated with an adjusted odds ratio (OR) for breast cancer of 1.26 (95% confidence interval [CI], 1.24–1.29), while ET had an OR of 1.06 (95% CI, 1.03–1.10). In women aged 50 to 59 who used EPT for 5 years or more, 15 additional breast cancers were diagnosed per 10,000 woman-years; for ET users, the attributable risk was 3. Although risk rose with longer HT duration, this trend was less evident with ET than EPT.
In addition, the increased risk associated with ET use was less pronounced in women with a BMI greater than 30 kg/m2. Among EPT users, risks were similar with the progestins medroxyprogesterone acetate (MPA), norethindrone (NET), and levonorgestrel (LNG). Likewise, risks were similar regardless of estrogen dose and route of administration (that is, oral vs transdermal). Vaginal estrogen was not associated with a higher or lower risk for breast cancer. Among past users of ET or EPT (with MPA), no increased risk was noted 5 years or more after stopping HT. For users of EPT (with NET or LNG), risks diminished 5 years or more after stopping HT but remained modestly elevated compared with risk in never-users.4
In this large observational UK study, ET was associated with minimally elevated risk for breast cancer, while in the WHI study, ET reduced the risk for breast cancer. For EPT, the excess risk in both studies was identical. As the authors note, mean BMI in the UK study participants was slightly lower than that in the WHI participants, a distinction that might explain the differing findings with ET use.
In our practice, for women with an intact uterus who are considering the use of EPT for treatment of bothersome menopausal symptoms, we counsel that long-term use of HT slightly elevates the risk for breast cancer. By contrast, we advise posthysterectomy women with bothersome menopausal symptoms that ET does not appear to increase the risk for breast cancer.
Continue to: Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women...
Frequency of nonindicated BSO at the time of hysterectomy in pre- and perimenopausal women
Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2020;28:8-11. doi: 10.1097/GME.0000000000001652.
While prevention of ovarian cancer is an important benefit of bilateral salpingo-oophorectomy (BSO), performing a BSO at the time of hysterectomy in pre- or perimenopausal patients not only will induce surgical menopause but also is associated with significantly increased overall mortality and an increased risk of mortality due to cardiovascular disease in patients younger than age 45.5,6 Earlier BSO also has been associated with diabetes, accelerated bone density loss, sexual dysfunction, mood disorders, and decreased cognitive function.7
BSO at hysterectomy: How many procedures are not indicated?
To evaluate the prevalence and predictors of unnecessary BSO at the time of hysterectomy, Wong and colleagues conducted a multicenter retrospective review of hysterectomy procedures completed at 6 Canadian hospitals.8 Criteria for unnecessary BSO included age younger than 51 years; benign preoperative diagnosis (other than endometriosis, premenstrual dysphoric disorder, and gender dysphoria); and absence of endometriosis and pelvic adhesions.
A total of 2,656 hysterectomies were performed by 75 surgeons (28 fellowship trained and 47 generalists) across 3 community and 3 tertiary care hospitals between 2016 and 2018. At the time of hysterectomy, 749 patients (28%) underwent BSO. Of these, 509 women (68%) had at least 1 indication for concurrent BSO based on preoperative diagnosis.
Key study findings. Concurrent BSO procedures performed at academic hospitals were more likely to have a preoperative indication compared with BSO performed at community sites (70% vs 63%; OR, 1.42; 95% CI, 1.02–1.97; P = .04). BSO was more likely to be indicated when performed by fellowship-trained surgeons compared with surgeries performed by generalist surgeons (75% vs 63%; OR, 1.76; 95% CI, 1.26–2.44, P = .001). BSO procedures performed with vaginal hysterectomy were less likely to be indicated (3 of 20, 15%) when compared with open hysterectomy (74 of 154, 48%) and laparoscopic hysterectomy (432 of 575, 75%).
Of the patients who lacked a preoperative indication for concomitant BSO, 105 of 239 (43.9%) were younger than age 51. Overall, 8% (59 of 749) of patients in the study cohort had an unnecessary BSO based on a combination of preoperative diagnosis, age younger than age 51, and intraoperative factors including absence of endometriosis and adhesions.
The retrospective study by Wong and colleagues provides the first assessment of Canadian practice patterns with respect to concurrent BSO at the time of hysterectomy. The authors found that, overall, more than two-thirds of BSO procedures were indicated. However, the proportion of BSO that was indicated was higher in teaching hospitals and in surgeries performed by fellowship-trained gynecologists. These important observations underscore the role of clinician education in reducing nonindicated BSO in pre- and perimenopausal women undergoing hysterectomy for benign disease.
Continue to: HT for menopausal depression: Which patients may benefit?
HT for menopausal depression: Which patients may benefit?
Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686- 705. doi:10.1176/appi.ajp.2020.19080848.
The cumulative lifetime prevalence of major depression in US women is 21%.9 An increased risk of mood symptoms and major depressive disorder occurs with the cessation of ovarian hormone production during menopause. In a review of both physiology and clinical studies, an American Psychiatric Association task force found support for several hormone-related strategies for treating depression and highlighted the rapidly advancing, but mixed, findings in this field.10
Clinical trials that examined mood in peri- and postmenopausal women treated with HT have produced mixed results for a variety of reasons, including differences in psychiatric symptomatology across studies and differences in treatment timing in relation to menopause onset.
HT effectiveness for depression depends on menopausal status
Five studies included in a meta-analysis by Rubinow and colleagues examined the use of ET and EPT as antidepressant monotherapy in peri- or postmenopausal women with major depression.11 Of the 3 higher-quality studies, 2 conducted in perimenopausal women demonstrated the antidepressant efficacy of transdermal estrogen patches compared with placebo. The third study included a mixed population of both peri- and postmenopausal women, and it found that increased estradiol levels (spontaneously occurring or due to ET) were associated with improvement in depression in perimenopausal women but not in postmenopausal women.11
ET also has been investigated as a potential adjunctive treatment to selective serotonin reuptake inhibitors (SSRIs). In a retrospective analysis of a multicenter randomized controlled trial of fluoxetine in patients with depression, women who received ET and fluoxetine demonstrated a greater improvement than those who received fluoxetine monotherapy.12 One small study that prospectively assessed ET in combination with an antidepressant in postmenopausal women demonstrated no benefit of ET in treating depression.13 Another small trial found that while combining transdermal ET with an SSRI accelerated symptom improvement, by the end of the 10-week study, treatment efficacy in the HT plus SSRI group was no greater than that observed in the SSRI-only group.14
Nineteen studies included in the metaanalysis by Rubinow and colleagues, which examined mood after ET or EPT treatment in nondepressed women, found little evidence of benefit, particularly in women without other physical symptoms of menopause.11
The Kronos Early Estrogen Prevention Study (KEEPS) followed 661 women who received either oral estrogen plus progesterone, transdermal estrogen plus progesterone, or placebo over 4 years.15 Women with clinical depression were excluded from the study; however, women with mild to moderate mood symptoms who were being treated with an antidepressant were included. Improvements in depressive symptoms and anxiety were observed only in the oral estrogen plus progesterone group compared with the placebo group.15
In a study of 172 euthymic peri- and postmenopausal women treated for 12 months with transdermal estrogen plus oral progesterone, investigators found that, unlike postmenopausal women and those in the late perimenopausal transition, only women in the early perimenopausal transition had a lower risk of developing depressive symptoms.16
Bottom line
This complex literature suggests that ET/HT interventions are most likely to be successful when implemented early in the menopausal transition. The clearest indication for the use of HT is for perimenopausal women experiencing depression who are also experiencing menopausal symptoms (for example, bothersome hot flashes). There is little evidence that the use of ET/HT in late perimenopausal or postmenopausal women effectively treats depression; accordingly, HT is not recommended for the treatment of mood disorders in this population. The more ambiguous cases are those of perimenopausal women who are depressed but do not have classic vasomotor symptoms; some evidence supports the antidepressant efficacy of HT in this setting.11 Although some studies suggest that HT can be effective in preventing depression in perimenopausal women, more evidence is needed.16
A trial of ET/EPT is reasonable in perimenopausal women with depression and classic menopausal symptoms. Use of HT also can be considered either alone or in combination with an SSRI in perimenopausal women with depression who do not have significant classic menopausal symptoms. However, HT is not recommended as prophylaxis against depression in euthymic perimenopausal women. Finally, keep in mind that the use of HT to address mood issues constitutes off-label use.
The menopause transition: A key period for strategizing CVD risk factor reduction

Chrisandra L. Shufelt, MD, MS, NCMP
Dr. Shufelt is Associate Director of the Barbra
Streisand Women’s Heart Center, Smidt
Heart Institute, Cedars-Sinai Medical Center,
Los Angeles, California.

JoAnn E. Manson, MD, DrPH, NCMP
Dr. Manson is Professor of Medicine and the
Michael and Lee Bell Professor of Women’s
Health at Harvard Medical School; Professor
in the Department of Epidemiology, Harvard
T.H. Chan School of Public Health; and Chief
of the Division of Preventive Medicine
at Brigham and Women’s Hospital, Boston,
Massachusetts.
The authors report no financial relationships relevant to this article. Dr. Manson is a coauthor of the AHA Scientific Statement discussed in this article.
In the United States, nearly one-half of a woman’s life, on average, will be lived after menopause. For women with natural menopause, the menopause transition (MT) can begin 2 to 7 years before and may extend 1 year past the final menstrual period, which occurs at an average age of 51 years. For women with surgical menopause, the MT occurs abruptly with the sudden loss of endogenous ovarian hormones. Both types of transitions mark a critical time period when reproduction and endogenous sex hormone levels diminish and when cardiovascular disease (CVD) risk factors begin to rise.
The 2020 American Heart Association (AHA) scientific statement, “Menopause transition and cardiovascular disease risk: Implications for timing of early prevention,” highlights the MT as a window of opportunity for CVD prevention.1
CVD risk factors associated with ovarian aging
In the AHA scientific statement, data from several longitudinal women’s health studies were used to identify which CVD risk factor changes during the MT are related to ovarian aging as opposed to chronologic aging. Independent of aging, those associated with reproductive or ovarian aging included an increase in serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and apolipoprotein B. Changes in high-density lipoprotein cholesterol (HDL-C) particles and function also occur during the MT, which may explain why higher HDL-C levels during the MT and the postmenopausal years are not as cardioprotective as during the premenopausal period.
Changes in body composition and adipose tissue distribution also are associated with ovarian aging, with reduction in muscle mass and lean body mass and an increase in abdominal/visceral fat and subcutaneous adipose tissue. Although these body composition changes reflect ovarian aging, midlife weight gain is more closely related to chronologic aging.
The risk of the metabolic syndrome constellation of risk factors was found to be more closely associated with ovarian aging, whereas changes in blood pressure, insulin, and glucose individually tracked more closely with chronologic aging. Additionally, the AHA statement notes the research that identified several symptoms during the MT—including vasomotor symptoms, sleep disturbance, and depression—as being associated with more adverse CVD risk factor status and with subclinical measures of atherosclerosis. Additional research on the mechanistic basis for these associations is needed.
Chronologic age and type of menopause
Notably, a woman’s age and type of menopause matter with respect to CVD risk. Higher CVD risk is seen in women with premature onset (age < 40 years) or early onset (age < 45 years) of menopause and in women undergoing surgical menopause (bilateral oophorectomy) before age 45. In general, menopausal hormone therapy (HT) is recommended for women with premature or early menopause, whether natural or surgical, with continuation through at least the average age of natural menopause. In other women, although not recommended for the express purpose of CVD prevention, menopausal HT is appropriate for the treatment of bothersome vasomotor or other menopausal symptoms, especially when therapy is started before age 60 or within 10 years of menopause among women who are not at elevated risk of CVD.
While the AHA statement suggests that some women who begin estrogen early in menopause may experience reduced coronary heart disease risk, major research gaps remain with regard to HT dose, formulation, route of delivery, and recommended duration of treatment.
An opportunity to promote healthy lifestyle behaviors
Translating the AHA’s first-of-its-kind scientific statement into clinical practice requires recognition and awareness of the MT as a unique phase in a woman’s life associated with myriad changes in CVD risk factors. The statement underscores that the MT is an important time to target behavioral changes to promote CVD risk reduction, including lifestyle modifications in the AHA’s Life’s Simple 7 components (increased physical activity, smoking cessation, healthy diet, avoidance of weight gain) as well as vigilant control of blood pressure, cholesterol, and glucose levels. The MT is truly a window of opportunity for reinvigorated efforts to lower women’s CVD risk. ●
Reference
1. El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532. doi: 10.1161/CIR.000000000000912.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353- 1368. doi: 10.1001/jama.2013.278040.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Minami CA, Freedman RA. Menopausal hormone therapy and long-term breast cancer risk: further data from the Women’s Health Initiative trials. JAMA. 2020;324:347-349. doi: 10.1001/jama.2020.9620.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279. doi: 10.1016/j.ajog.2017.07.037.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15-23. doi: 10.1097/gme.0b013e31818888f7.
- Karp NE, Fenner DE, Burgunder-Zdravkovski L, et al. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstetr Gynecol. 2015;213:716.e1-6. doi: 10.1016/j.ajog.2015.05.062.
- Wong J, Murji A, Sunderji Z, et al. Unnecessary bilateral salpingo-oophorectomy at the time of hysterectomy and potential for ovarian preservation. Menopause. 2021;28:8-11. doi: 10.1097/GME.0000000000001652.
- Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity, and recurrence. J Affect Disord. 1993;29:85- 96. doi: 10.1016/0165-0327(93)00026-g.
- Dwyer JB, Aftab A, Radhakrishnan R, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal treatments for major depressive disorder: state of the art. Am J Psychiatry. 2020;177:686-705. doi:10.1176/appi. ajp.2020.19080848.
- Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32:539-549. doi: 10.1002/ da.22391.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Dias RS, Kerr-Corrêa F, Moreno RA, et al. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause. 2006;13:202-211. doi:10.1097/01.gme.0000198491.34371.9c.
- Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338- 343. doi: 10.1016/j.jpsychires.2006.03.009.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med. 2015;12:e1001833. doi: 10.1371/journal.pmed.1001833.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75:149–157. doi:10.1001/jamapsychiatry.2017.3998.
Clinical Edge Journal Scan Commentary: Contraception May 2021
Immediate postpartum long-acting reversible contraception (LARC) represents a safe and effective contraceptive strategy. Despite national guidelines recommending universal patient access, hospitals face significant barriers to offering inpatient LARCs. It is unclear why some hospitals successfully implement immediate postpartum LARCs, while others do not.
Moniz et al conducted a comparative, multiple case study of immediate postpartum LARC implementation at eleven “early adopter” U.S. hospitals and analyzed each hospital’s implementation strategy to produce generalizable knowledge about how and under what circumstances implementation of immediate postpartum LARCs unfold successfully. Between 2017-2018, the authors conducted single-day site visits and 78 semi-structured interviews with a variety of stakeholders (clinician champions, nurses, pharmacists, revenue cycle staff, and hospital administration). On average, sites used 18 (range 11-22) implementation strategies, including assessing institutional readiness for, and barriers to, implementation of immediate postpartum LARC, engaging reproductive justice experts and community resources to address social determinants of health, involving patients in implementation planning, and developing quality monitoring processes to evaluate clinical processes and outcomes. The researchers found that successful implementation of immediate postpartum LARC required three essential conditions: effective implementation champions who are supported by a multidisciplinary implementation team; creating an enabling financial environment; and engaging hospital administration. Additional findings from this study call for more support for individuals leading change in complex care settings, intentionally designing implementation interventions that take into account local contextual influences, and meaningfully engaging patients in the implementation process.
Of postpartum women, 61% in low- and middle-income countries (LMIC) have an unmet contraceptive need and many face high rates of short interpregnancy intervals (Moore). Additionally, 51%-96% of postpartum women in LMIC use short-acting methods of contraception (Moore), further highlighting the need for increased access to immediate postpartum LARC in LMIC. Data on the use and continuation of immediate postpartum LARC in LMIC is limited. Marchin et al conducted a systematic review and meta-analysis to determine 6-month continuation rates of immediate postpartum LARCs among women in 69 low-income countries that were enrolled in the Family Planning 2020 initiative. The meta-analysis ultimately focused on the copper IUD due to the absence of relevant studies on other LARC methods. The meta-analysis of 12 studies resulted in a pooled 6-month continuation rate for immediate postpartum copper IUDs of 87%, a rate comparable to continuation rates found in higher-income countries. This estimate had significant heterogeneity between studies. Secondary outcomes of expulsion, removal, and infection rates were low at 6%, 5%, and 0.2% respectively. High 6-month continuation rates and a low rate of adverse outcomes suggest immediate postpartum copper IUD insertion represents a feasible and acceptable postpartum contraceptive option for women living in LMIC.
In cases where contraception methods fail, are used incorrectly, or are not used at all, emergency contraception (EC) can be used after intercourse to prevent pregnancy. Timely access to, and accurate knowledge of, EC are especially important for rural women who are more likely to experience an unintended pregnancy resulting in a live birth compared to urban women. Milkowski et al analyzed publicly available data from the National Survey of Family Growth to estimate differences in oral EC use, access, and counseling by rural-urban county of residence among U.S. women aged 15-44 years. 10% of rural and 19% of urban women who had ever been sexually active reported ever using EC pills. Over the course of the study period (2006-2017), the percentage of women reporting ever-use of EC increased linearly in both rural and urban populations, with the prevalence of EC ever-use more than doubling in each group. This observation likely reflects an overall increase in EC use during a time period in which the federal government enacted several policies to improve access to EC. The study findings also highlight the need for improved patient counseling on EC. While the overall prevalence of EC counseling was low among all women, rural women were less likely to have received counseling on EC when compared to urban women.
Although many studies report weight gain in users of progestin-only hormonal contraception (POC), a recent Cochrane systematic review found that there was insufficient evidence to determine the effect of POCs on weight (Lopez). Beksinska et al conducted a secondary analysis of prospective weight change among women enrolled in the Evidence for Contraceptive options and HIV Outcomes (ECHO) trial, which was an open label, prospective, randomized multicenter trial that compared the risk of HIV acquisition among women randomized to injectable contraception (DMPA), the copper IUD, or a second generation two rod levonorgestrel (LNG) implant JadelleÒ. This trial was conducted at 12 sites across four African countries between 2015 and 2018. Eligible study participants were nonpregnant, HIV negative, sexually active women aged 16-35 years who desired contraception. The final sample size included 7,014 women randomly assigned to receive DMPA (2,293), the LNG implant (2,372), or the copper IUD (2,349). Using a standardized protocol and calibrated equipment across all study sites, weight and height were measured at baseline and at study exit at 12, 15, or 18 months. The mean weight increased amongst all three contraceptive groups and was significantly different in magnitude, with the largest gain in the DMPA group (3.5 kg), 2.4 kg in the LNG implant group, and 1.5 kg in the copper IUD group. Unlike copper IUD users, women in the DMPA and LNG implant group continued to gain weight after 1 year of contraceptive use. It is noteworthy that, regardless of contraceptive method allocation, not all women gained weight and a small proportion of women lost weight. When choosing a contraceptive method, women using POCs should be counselled about the potential side effect of weight gain.
References:
Moniz MH, Bonawitz K, Wetmore MK, et al. Implementing immediate postpartum contraception: a comparative case study at 11 hospitals. Implement Sci Commun. 2021;2(1):42. Published 2021 Apr 12.
Moore Z, Pfitzer A, Gubin R, et al. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 92 (2015): 31–39.
Marchin A, Moss A, Harrison M. A Meta-Analysis of Postpartum Copper IUD Continuation Rates in Low- and Middle-Income Countries. J Womens Health Dev. 2021;4(1):36-46.
Milkowski CM, Ziller EC, Ahrens KA. Rural-urban residence and emergency contraception use, access, and counseling in the United States, 2006-2017. Contracept X. 2021;3:100061.
Lopez L.M., Ramesh S., Chen M. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD008815.pub2.
Beksinska et al. “Weight change among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: Findings from a randomised, multicentre, open-label trial. EClinicalMedicine. 2021;34:100800.
Immediate postpartum long-acting reversible contraception (LARC) represents a safe and effective contraceptive strategy. Despite national guidelines recommending universal patient access, hospitals face significant barriers to offering inpatient LARCs. It is unclear why some hospitals successfully implement immediate postpartum LARCs, while others do not.
Moniz et al conducted a comparative, multiple case study of immediate postpartum LARC implementation at eleven “early adopter” U.S. hospitals and analyzed each hospital’s implementation strategy to produce generalizable knowledge about how and under what circumstances implementation of immediate postpartum LARCs unfold successfully. Between 2017-2018, the authors conducted single-day site visits and 78 semi-structured interviews with a variety of stakeholders (clinician champions, nurses, pharmacists, revenue cycle staff, and hospital administration). On average, sites used 18 (range 11-22) implementation strategies, including assessing institutional readiness for, and barriers to, implementation of immediate postpartum LARC, engaging reproductive justice experts and community resources to address social determinants of health, involving patients in implementation planning, and developing quality monitoring processes to evaluate clinical processes and outcomes. The researchers found that successful implementation of immediate postpartum LARC required three essential conditions: effective implementation champions who are supported by a multidisciplinary implementation team; creating an enabling financial environment; and engaging hospital administration. Additional findings from this study call for more support for individuals leading change in complex care settings, intentionally designing implementation interventions that take into account local contextual influences, and meaningfully engaging patients in the implementation process.
Of postpartum women, 61% in low- and middle-income countries (LMIC) have an unmet contraceptive need and many face high rates of short interpregnancy intervals (Moore). Additionally, 51%-96% of postpartum women in LMIC use short-acting methods of contraception (Moore), further highlighting the need for increased access to immediate postpartum LARC in LMIC. Data on the use and continuation of immediate postpartum LARC in LMIC is limited. Marchin et al conducted a systematic review and meta-analysis to determine 6-month continuation rates of immediate postpartum LARCs among women in 69 low-income countries that were enrolled in the Family Planning 2020 initiative. The meta-analysis ultimately focused on the copper IUD due to the absence of relevant studies on other LARC methods. The meta-analysis of 12 studies resulted in a pooled 6-month continuation rate for immediate postpartum copper IUDs of 87%, a rate comparable to continuation rates found in higher-income countries. This estimate had significant heterogeneity between studies. Secondary outcomes of expulsion, removal, and infection rates were low at 6%, 5%, and 0.2% respectively. High 6-month continuation rates and a low rate of adverse outcomes suggest immediate postpartum copper IUD insertion represents a feasible and acceptable postpartum contraceptive option for women living in LMIC.
In cases where contraception methods fail, are used incorrectly, or are not used at all, emergency contraception (EC) can be used after intercourse to prevent pregnancy. Timely access to, and accurate knowledge of, EC are especially important for rural women who are more likely to experience an unintended pregnancy resulting in a live birth compared to urban women. Milkowski et al analyzed publicly available data from the National Survey of Family Growth to estimate differences in oral EC use, access, and counseling by rural-urban county of residence among U.S. women aged 15-44 years. 10% of rural and 19% of urban women who had ever been sexually active reported ever using EC pills. Over the course of the study period (2006-2017), the percentage of women reporting ever-use of EC increased linearly in both rural and urban populations, with the prevalence of EC ever-use more than doubling in each group. This observation likely reflects an overall increase in EC use during a time period in which the federal government enacted several policies to improve access to EC. The study findings also highlight the need for improved patient counseling on EC. While the overall prevalence of EC counseling was low among all women, rural women were less likely to have received counseling on EC when compared to urban women.
Although many studies report weight gain in users of progestin-only hormonal contraception (POC), a recent Cochrane systematic review found that there was insufficient evidence to determine the effect of POCs on weight (Lopez). Beksinska et al conducted a secondary analysis of prospective weight change among women enrolled in the Evidence for Contraceptive options and HIV Outcomes (ECHO) trial, which was an open label, prospective, randomized multicenter trial that compared the risk of HIV acquisition among women randomized to injectable contraception (DMPA), the copper IUD, or a second generation two rod levonorgestrel (LNG) implant JadelleÒ. This trial was conducted at 12 sites across four African countries between 2015 and 2018. Eligible study participants were nonpregnant, HIV negative, sexually active women aged 16-35 years who desired contraception. The final sample size included 7,014 women randomly assigned to receive DMPA (2,293), the LNG implant (2,372), or the copper IUD (2,349). Using a standardized protocol and calibrated equipment across all study sites, weight and height were measured at baseline and at study exit at 12, 15, or 18 months. The mean weight increased amongst all three contraceptive groups and was significantly different in magnitude, with the largest gain in the DMPA group (3.5 kg), 2.4 kg in the LNG implant group, and 1.5 kg in the copper IUD group. Unlike copper IUD users, women in the DMPA and LNG implant group continued to gain weight after 1 year of contraceptive use. It is noteworthy that, regardless of contraceptive method allocation, not all women gained weight and a small proportion of women lost weight. When choosing a contraceptive method, women using POCs should be counselled about the potential side effect of weight gain.
References:
Moniz MH, Bonawitz K, Wetmore MK, et al. Implementing immediate postpartum contraception: a comparative case study at 11 hospitals. Implement Sci Commun. 2021;2(1):42. Published 2021 Apr 12.
Moore Z, Pfitzer A, Gubin R, et al. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 92 (2015): 31–39.
Marchin A, Moss A, Harrison M. A Meta-Analysis of Postpartum Copper IUD Continuation Rates in Low- and Middle-Income Countries. J Womens Health Dev. 2021;4(1):36-46.
Milkowski CM, Ziller EC, Ahrens KA. Rural-urban residence and emergency contraception use, access, and counseling in the United States, 2006-2017. Contracept X. 2021;3:100061.
Lopez L.M., Ramesh S., Chen M. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD008815.pub2.
Beksinska et al. “Weight change among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: Findings from a randomised, multicentre, open-label trial. EClinicalMedicine. 2021;34:100800.
Immediate postpartum long-acting reversible contraception (LARC) represents a safe and effective contraceptive strategy. Despite national guidelines recommending universal patient access, hospitals face significant barriers to offering inpatient LARCs. It is unclear why some hospitals successfully implement immediate postpartum LARCs, while others do not.
Moniz et al conducted a comparative, multiple case study of immediate postpartum LARC implementation at eleven “early adopter” U.S. hospitals and analyzed each hospital’s implementation strategy to produce generalizable knowledge about how and under what circumstances implementation of immediate postpartum LARCs unfold successfully. Between 2017-2018, the authors conducted single-day site visits and 78 semi-structured interviews with a variety of stakeholders (clinician champions, nurses, pharmacists, revenue cycle staff, and hospital administration). On average, sites used 18 (range 11-22) implementation strategies, including assessing institutional readiness for, and barriers to, implementation of immediate postpartum LARC, engaging reproductive justice experts and community resources to address social determinants of health, involving patients in implementation planning, and developing quality monitoring processes to evaluate clinical processes and outcomes. The researchers found that successful implementation of immediate postpartum LARC required three essential conditions: effective implementation champions who are supported by a multidisciplinary implementation team; creating an enabling financial environment; and engaging hospital administration. Additional findings from this study call for more support for individuals leading change in complex care settings, intentionally designing implementation interventions that take into account local contextual influences, and meaningfully engaging patients in the implementation process.
Of postpartum women, 61% in low- and middle-income countries (LMIC) have an unmet contraceptive need and many face high rates of short interpregnancy intervals (Moore). Additionally, 51%-96% of postpartum women in LMIC use short-acting methods of contraception (Moore), further highlighting the need for increased access to immediate postpartum LARC in LMIC. Data on the use and continuation of immediate postpartum LARC in LMIC is limited. Marchin et al conducted a systematic review and meta-analysis to determine 6-month continuation rates of immediate postpartum LARCs among women in 69 low-income countries that were enrolled in the Family Planning 2020 initiative. The meta-analysis ultimately focused on the copper IUD due to the absence of relevant studies on other LARC methods. The meta-analysis of 12 studies resulted in a pooled 6-month continuation rate for immediate postpartum copper IUDs of 87%, a rate comparable to continuation rates found in higher-income countries. This estimate had significant heterogeneity between studies. Secondary outcomes of expulsion, removal, and infection rates were low at 6%, 5%, and 0.2% respectively. High 6-month continuation rates and a low rate of adverse outcomes suggest immediate postpartum copper IUD insertion represents a feasible and acceptable postpartum contraceptive option for women living in LMIC.
In cases where contraception methods fail, are used incorrectly, or are not used at all, emergency contraception (EC) can be used after intercourse to prevent pregnancy. Timely access to, and accurate knowledge of, EC are especially important for rural women who are more likely to experience an unintended pregnancy resulting in a live birth compared to urban women. Milkowski et al analyzed publicly available data from the National Survey of Family Growth to estimate differences in oral EC use, access, and counseling by rural-urban county of residence among U.S. women aged 15-44 years. 10% of rural and 19% of urban women who had ever been sexually active reported ever using EC pills. Over the course of the study period (2006-2017), the percentage of women reporting ever-use of EC increased linearly in both rural and urban populations, with the prevalence of EC ever-use more than doubling in each group. This observation likely reflects an overall increase in EC use during a time period in which the federal government enacted several policies to improve access to EC. The study findings also highlight the need for improved patient counseling on EC. While the overall prevalence of EC counseling was low among all women, rural women were less likely to have received counseling on EC when compared to urban women.
Although many studies report weight gain in users of progestin-only hormonal contraception (POC), a recent Cochrane systematic review found that there was insufficient evidence to determine the effect of POCs on weight (Lopez). Beksinska et al conducted a secondary analysis of prospective weight change among women enrolled in the Evidence for Contraceptive options and HIV Outcomes (ECHO) trial, which was an open label, prospective, randomized multicenter trial that compared the risk of HIV acquisition among women randomized to injectable contraception (DMPA), the copper IUD, or a second generation two rod levonorgestrel (LNG) implant JadelleÒ. This trial was conducted at 12 sites across four African countries between 2015 and 2018. Eligible study participants were nonpregnant, HIV negative, sexually active women aged 16-35 years who desired contraception. The final sample size included 7,014 women randomly assigned to receive DMPA (2,293), the LNG implant (2,372), or the copper IUD (2,349). Using a standardized protocol and calibrated equipment across all study sites, weight and height were measured at baseline and at study exit at 12, 15, or 18 months. The mean weight increased amongst all three contraceptive groups and was significantly different in magnitude, with the largest gain in the DMPA group (3.5 kg), 2.4 kg in the LNG implant group, and 1.5 kg in the copper IUD group. Unlike copper IUD users, women in the DMPA and LNG implant group continued to gain weight after 1 year of contraceptive use. It is noteworthy that, regardless of contraceptive method allocation, not all women gained weight and a small proportion of women lost weight. When choosing a contraceptive method, women using POCs should be counselled about the potential side effect of weight gain.
References:
Moniz MH, Bonawitz K, Wetmore MK, et al. Implementing immediate postpartum contraception: a comparative case study at 11 hospitals. Implement Sci Commun. 2021;2(1):42. Published 2021 Apr 12.
Moore Z, Pfitzer A, Gubin R, et al. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 92 (2015): 31–39.
Marchin A, Moss A, Harrison M. A Meta-Analysis of Postpartum Copper IUD Continuation Rates in Low- and Middle-Income Countries. J Womens Health Dev. 2021;4(1):36-46.
Milkowski CM, Ziller EC, Ahrens KA. Rural-urban residence and emergency contraception use, access, and counseling in the United States, 2006-2017. Contracept X. 2021;3:100061.
Lopez L.M., Ramesh S., Chen M. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD008815.pub2.
Beksinska et al. “Weight change among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: Findings from a randomised, multicentre, open-label trial. EClinicalMedicine. 2021;34:100800.
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).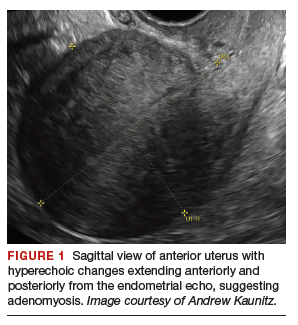
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
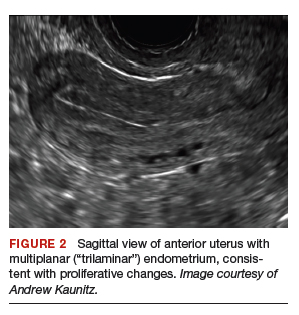

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
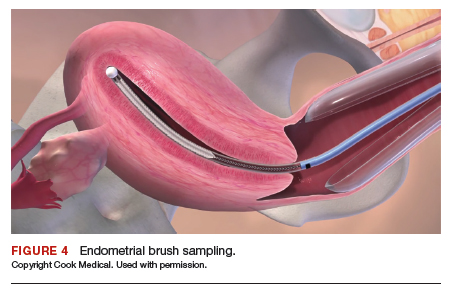
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
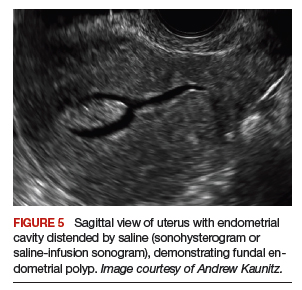
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
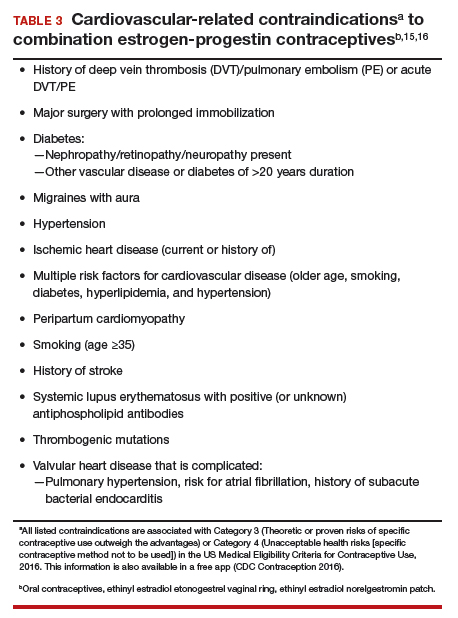
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.