User login
In reply: Measles: More than the rash
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
In Reply: We thank Dr. Cunha for his comments and appreciate the opportunity to emphasize important points that he highlights.
We agree that measles is a systemic illness with important extradermatologic manifestations that are critical to the diagnosis, and that the nondermatologic manifestations often precede the rash and serve to distinguish measles from other systemic illnesses. As discussed in our review, the respiratory prodrome of cough, coryza, and conjunctivitis is very distinctive and serves as an important clue to the diagnosis. Likewise, we acknowledge the importance of gastrointestinal findings in measles and note appendicitis as an important complication that is well described. Although Koplik spots are pathognomonic, we do stress that these often are not present at the time of presentation.
Finally, we agree that measles is a clinical diagnosis, and that the clinical manifestations beyond the dermatologic manifestations noted in our review and highlighted by Dr. Cunha are extremely helpful to the clinician in considering the diagnosis.
Measles: Back again
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
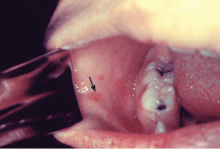
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
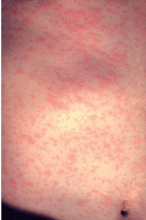
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
Measles continues to rear its head in the United States. Because it is so contagious, even the few cases introduced by travelers quickly spread to susceptible contacts. Life-threatening and severely disabling complications can occur, although this is rare. Widespread immunization and prompt recognition and isolation of contacts are key to controlling outbreaks.
This article reviews the epidemiology of measles, describes its distinctive clinical picture, and provides recommendations for infection control and prevention, including in immunosuppressed populations.
MEASLES IS SERIOUS AND HIGHLY CONTAGIOUS
Up to 90% of susceptible people develop measles after exposure, making it one of the most contagious of infections. The virus is transmitted by airborne spread when an infected person coughs or sneezes, or by direct contact with infectious droplets. The virus can remain infectious in the air or on a surface for up to 2 hours.1
Worldwide, an estimated 20 million people are infected with measles each year, and 146,000 die of complications. In 1980, before widespread vaccination, 2.6 million deaths were attributable to measles annually. In the United States before the introduction of measles vaccine in 1963, measles was a significant cause of disease and death: an estimated 3 to 4 million people were infected annually, although only about 549,000 were reported. There were 48,000 hospitalizations, 1,000 cases of permanent brain damage from measles encephalitis, and 495 deaths annually.2
Outbreaks still occur regularly
In 2000, measles was declared eliminated from the United States,3 but annual outbreaks have occurred since then as a result of cases imported from other countries and their subsequent transmission to unvaccinated people. From 2001 to 2012, a median of four outbreaks and 60 cases were reported annually to the US Centers for Disease Control and Prevention.4
In January 2015, a multistate measles outbreak originating in Disneyland in California was recognized. As of April 17, when the outbreak was declared over, 111 measles cases from seven states had been linked to this outbreak.5 Of the evaluable cases, 44% were in unvaccinated people and 38% were in those whose vaccination status was unknown or undocumented. The median age of patients was 21, and 20% required hospitalization.
This outbreak, as well as four other smaller US outbreaks the same year, underscores the transmissibility of the virus in populations containing only a small percentage of unvaccinated people.6
DISTINCTIVE CLINICAL PICTURE
The incubation period for measles infection is 7 to 21 days, with most cases becoming apparent 10 to 12 days after exposure. Measles should be suspected in a patient with the following clinical features whose history indicates susceptibility and exposure (ie, an unimmunized person with a history of exposure or travel):
Severe acute respiratory illness. Measles usually presents as an acute respiratory viral illness, which typically lasts 2 to 4 days. The illness involves high fevers, malaise, anorexia, and the “three Cs”: cough, coryza (rhinitis), and conjunctivitis. Patients usually appear sicker than those with more common viral illnesses.
Koplik spots, which are pathognomonic for measles, are seen in the first few days of illness. They are bluish-white, slightly raised lesions on an erythematous base on the buccal mucosa, usually opposite the first molar (Figure 1). Spots can also be seen on the soft palate, conjunctiva, and vaginal mucosa. Koplik spots usually disappear after a few days and often are not appreciable at the time of evaluation.
Discrete erythematous patches develop on the face and neck a day after the appearance of Koplik spots. This rash becomes more confluent as it spreads to involve the entire body (Figure 2). It typically lasts for 3 to 7 days, then fades in a similar pattern. The confluent nature of this rash and its spread from the face and neck to the entire body are characteristic of measles. Patients are highly contagious from 4 days before the onset of the rash to 4 days after.
COMPLICATIONS CAN BE SEVERE
Those at highest risk for measles complications are infants, children under age 5, adults over age 20, pregnant women, and immunosuppressed individuals.7
Pneumonia—either a primary measles pneumonia or a secondary viral or bacterial pneumonia—is the most common cause of death.8,9 Viruses complicating measles are typically adenovirus and herpes simplex virus. Bacteria causing secondary infection are usually Staphylococcus aureus and Streptococcus pneumoniae and, less commonly, gram-negative bacteria.
Laryngotracheobronchitis (croup) is the second most common cause of death, with bacteria and viruses similar to those causing measles-related pneumonia.
Otitis media is the most common complication of measles. Other respiratory complications include mastoiditis, pneumothorax, and mediastinal emphysema.
Acute measles encephalitis occurs in 1 measles case per 1,000 and often results in permanent brain damage. During the convalescent phase of the illness, fever again emerges, with the development of headaches, seizures, and altered consciousness.10
Subacute sclerosing panencephalitis is a rare fatal degenerative disease of the central nervous system caused by a persistent infection with a defective measles virus. The precise pathophysiology is unclear, but it is thought that mutations of the viral genome lead to altered cellular immunity.11 The condition typically occurs 7 to 10 years after the initial measles infection, particularly in those who developed measles before age 2. Clinical manifestations include behavioral disturbances, intellectual deterioration, and myoclonic seizures, slowly progressing to a vegetative state and death.12
Other complications of measles include diarrhea and stomatitis, which are associated with malnutrition in developing countries, and subclinical hepatitis, thrombocytopenia, appendicitis, ileocolitis, hypokalemia, and myocarditis.
During pregnancy, measles infection can be complicated by primary measles pneumonia and is associated with an increased risk of miscarriage and premature birth.13
Patients with a cell-mediated immunodeficiency who develop measles are particularly susceptible to fatal measles pneumonia and acute progressive encephalitis.14
ATYPICAL MEASLES IN THOSE WHO RECEIVED KILLED VACCINE
From 1963 to 1967, a killed measles vaccine was available in the United States. Those who received this vaccine are susceptible to an atypical form of measles when exposed to the virus,15 characterized by a 1- to 2-day prodrome, followed by the appearance of a maculopapular or petechial rash on the distal extremities that spreads centripetally. Patients develop high fever and edema of the hands and feet, and have a more prolonged course than with classic measles. It is believed not to be contagious.16
LABORATORY CONFIRMATION
Laboratory confirmation of measles is recommended for suspected cases. Because viral isolation is technically difficult and is not readily available in most laboratories, measles-specific immunoglobulin M antibody serologic testing is most commonly used. It is almost 100% sensitive when done 2 to 3 days after the onset of the rash.17
Measles RNA testing by real-time polymerase chain reaction to detect measles virus in the blood, throat, or urine is more specific and if available may be preferred over serologic testing.18
SUPPORTIVE MANAGEMENT AND VITAMIN A SUPPLEMENTATION
No specific antiviral therapy for measles is available. Management involves supportive measures and monitoring for secondary bacterial complications.
The World Health Organization and the American Academy of Pediatrics recommend vitamin A supplementation for all children with acute measles.19 In developing countries, it has been shown to reduce rates of morbidity and death in measles-infected children.20 In the United States, children with measles have been found to have low serum levels of vitamin A, with lower levels associated with more severe disease.
VACCINATION RECOMMENDATIONS
The only measles vaccine available in the United States is a live further-attenuated strain prepared in chick embryo cell culture and combined with mumps and rubella vaccine (MMR) or with measles, mumps, rubella, and varicella vaccine (MMRV).
Healthy children. Two doses of measles vaccine are recommended, as a single dose is associated with a 5% failure rate. The recommended schedule is:
- First dose at age 12 to 15 months
- Second dose at the time of school entry (ages 4 to 6), or at any time at least 28 days after the first dose.19
More than 99% of children who receive two doses of vaccine according to this schedule develop serologic evidence of measles immunity. Vaccination provides long-term immunity, and many epidemiologic studies have documented that waning immunity after vaccination occurs only very rarely.21
All school-age children, including elementary, middle, and high school students, who received only one dose of measles vaccine should receive the second dose.
Adults born in 1957 or later should receive at least one dose of measles vaccine unless they have other acceptable evidence of immunity, such as:4
- Documentation of age-appropriate live measles vaccine, ie, one dose of vaccine for adults not at high risk, or two doses for those at high risk (see below)
- Laboratory evidence of immunity (ie, measles immunoglobulin G in serum)
- Laboratory confirmation of disease.
Adults born before 1957 can be considered to be immune to measles, although MMR vaccine can be administered in those without contraindications.
Adults at increased risk of exposure or transmission of measles and who do not have evidence of immunity should receive two doses of MMR vaccine, given at least 28 days apart. This high-risk group includes:
- Students attending college or other post-high school educational institution
- Healthcare personnel
- International travelers.
During measles outbreaks, every effort should be made to ensure that those at high risk are vaccinated with two doses of MMR or have other acceptable evidence of immunity.
LIVE VACCINE IS SAFE FOR MOST PEOPLE
Mild side effects. A transient fever, which may be accompanied by a discrete or confluent rash, occurs in 5% to 15% of recipients 5 to 12 days after vaccination.
Transmission does not occur. People who have been newly vaccinated do not transmit the virus to susceptible contacts, even if they develop a vaccine-associated rash. The vaccine can safely be given to close contacts of immunocompromised and other susceptible people.
Egg allergy not a concern. Measles vaccine is produced in chick embryo cell culture but has been shown to be safe for people with egg allergy and is recommended without the need for egg allergy testing.19
Autism link debunked. No scientific evidence shows that the risk of autism is higher in children who receive MMR vaccine than in those who do not. In 2001, an Institute of Medicine report rejected a causal relationship between MMR vaccine and autism spectrum disorders.22
CONTRAINDICATIONS
Measles vaccine is contraindicated for:
- Patients who have cell-mediated immune deficiencies, except human immunodeficiency virus (HIV) infection
- Pregnant women
- Those who have had a severe allergic reaction to a vaccine component in the past
- Those with moderate or severe acute illness
- Those who have recently received immunoglobulin products.
People with HIV infection who are severely immunosuppressed should not receive live measles vaccine. However, because of the risk of severe measles in HIV-infected patients and because the vaccine has been shown to be safe for patients with HIV without severe immunosuppression, the vaccine is recommended for those with asymptomatic or mildly symptomatic HIV infection who do not have evidence of severe immunosuppression (ie, CD4 lymphocytes < 15% or < 200 cells/µL).3,4
INFECTION CONTROL AND PREVENTION
Healthcare workers should maintain a high index of suspicion for measles and implement isolation procedures promptly in patients with a febrile illness, rash, and a history of travel abroad or contact with travelers from abroad.23 Suspected cases should be reported promptly to local health agencies to help limit spread.
Patients with measles should be placed in airborne isolation (eg, use of an N95 or higher level respirator and an airborne infection isolation room) for 4 days after the onset of the rash in a normal host and for the duration of the illness in an immunocompromised patient. Healthcare staff, regardless of their immunity status, should adhere to these precautions when entering the room of infected patients.
Immunization programs should be established to ensure that everyone who works or volunteers in healthcare facilities is protected against measles.4
Postexposure prophylaxis. Measles vaccination given to susceptible contacts within 72 hours of exposure may provide protection against infection and induces protection against subsequent measles exposures.24,25
Vaccination is the best intervention for susceptible contacts older than 12 months who do not have a contraindication to measles vaccination, and for those who have received only one dose of measles vaccine.
Passive immunization. Active immunization is the best strategy for controlling measles outbreaks. Passive immunization with intramuscularly or intravenously administered immunoglobulin given within 6 days of exposure can be used to prevent transmission or modify the clinical course of infection for susceptible contacts at high risk of developing severe or fatal measles. This includes people who are being treated with immunosuppressive agents, HIV-infected, pregnant, or younger than 1 year of age.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
- Stokes J Jr, Reilly CM, Buynak EB, Hilleman MR. Immunologic studies of measles. Am J Hyg 1961; 74:293–303.
- Bloch AB, Orenstein WA, Stetler HC, et al. Health impact of measles vaccination in the United States. Pediatrics 1985; 76:524–532.
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis 2004; 189(suppl 1):S43–S47.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention (CDC). Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34.
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4 - April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373–376.
- Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis 2004; 189(suppl 1):S27–S35.
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189(suppl 1):S4–S16).
- Gremillion DH, Crawford GE. Measles pneumonia in young adults. An analysis of 106 cases. Am J Med 1981; 71:539–542.
- Quiambao BP, Gatchalian SR, Halonen P, et al. Coinfection is common in measles-associated pneumonia. Pediatr Infect Dis J 1998; 17:89–93.
- Johnson RT, Griffin D, Hirsch R, et al. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med 1984; 310:137–141.
- Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J 2002; 78:63–70.
- Sever JL. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467–473.
- Atmar RL, Englund JA, Hammill H. Complications of measles during pregnancy. Clin Infect Dis 1992; 14:217–226.
- Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA 1992; 267:1237–1241.
- Frey HM, Krugman S. Atypical measles syndrome: unusual hepatic, pulmonary, and immunologic aspects. Am J Med Sci 1981; 281:51–55.
- Fulginiti VA, Eller JJ, Downie AW, Kempe CH. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 1967; 202:1075–1080.
- Bellini WJ, Helfand RF. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003; 187(suppl 1):S283–S290.
- Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 2001; 39:375–376.
- Measles. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489-499.
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005; 4:CD001479.
- Markowitz LE, Preblud SR, Fine PE, Orenstein WA. Duration of live measles vaccine-induced immunity. Pediatr Infect Dis J 1990; 9:101–110.
- Stratton K, Gable A, Shetty P, McCormick M. Immunization safety review: measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press; 2001.
- Centers for Disease Control and Prevention (CDC). 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/hicpac/2007ip/2007isolationprecautions.html. Accessed April 8, 2016.
- Berkovich S, Starr S. Use of live-measles-virus vaccine to abort an expected outbreak of measles within a closed population. N Engl J Med 1963; 269:75–77.
- Ruuskanen O, Salmi TT, Halonen P. Measles vaccination after exposure to natural measles. J Pediatr 1978; 93:43–46.
KEY POINTS
- Patients with measles are usually very sick with high fever, cough, rhinitis, and conjunctivitis.
- Koplik spots—small bluish-white lesions on the buccal mucosa—are usually evident only in the first few days of illness. Soon after, a patchy red rash develops, starting with the face and neck, then spreading to the entire body.
- Measles can lead to pneumonia, encephalitis, brain damage, and death.
- Suspected cases should be isolated and susceptible contacts vaccinated or given immunoglobulin if at high risk of developing severe disease.
- The diagnosis should be confirmed by serologic testing with measles-specific immunoglobulin M antibody.
- Vaccination confers lifelong immunity and is recommended for all healthy children in two doses: the first at 12 to 15 months of age and the second at the time of school entry.