User login
Bosentan Reduced Occurrence of Digital Ulcers in Systemic Sclerosis
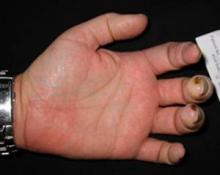
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Major Finding: Treatment with bosentan reduced the occurrence of new digital ulcers in SSc patients who had multiple ulcers at treatment initiation by 30%.
Data Source: A randomized, double-blind, placebo-controlled study comprising 188 systemic sclerosis patients.
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Bosentan Reduced Occurrence of Digital Ulcers in Systemic Sclerosis
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown. The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo. Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline. The study’s primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. “Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers,” they stated. “This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect.”
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, “the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline.”
The reduction of new digital ulcers in bosentan-treated patients “did not translate into a smaller ulcer burden, as was seen in the previous study,” the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote. Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing “the need for continual monitoring of liver function with this treatment,” the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc “may be challenged,” the authors wrote. “In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up.” However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment “may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers].”
This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown. The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo. Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline. The study’s primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. “Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers,” they stated. “This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect.”
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, “the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline.”
The reduction of new digital ulcers in bosentan-treated patients “did not translate into a smaller ulcer burden, as was seen in the previous study,” the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote. Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing “the need for continual monitoring of liver function with this treatment,” the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc “may be challenged,” the authors wrote. “In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up.” However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment “may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers].”
This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown. The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo. Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline. The study’s primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. “Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers,” they stated. “This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect.”
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, “the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline.”
The reduction of new digital ulcers in bosentan-treated patients “did not translate into a smaller ulcer burden, as was seen in the previous study,” the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote. Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing “the need for continual monitoring of liver function with this treatment,” the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc “may be challenged,” the authors wrote. “In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up.” However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment “may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers].”
This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Major Finding: Treatment with bosentan reduces the occurrence of new digital ulcers in SSc patients who have multiple ulcers at treatment initiation.
Data Source: Treatment with bosentan reduced new ischemic digital ulcers by 30% in a randomized, double-blind, placebo-controlled study comprising 188 systemic sclerosis patients.
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Hearing Loss in Pneumococcal Meningitis Linked to Otitis, Serotype 9V Infection
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
Hearing Loss in Pneumococcal Meningitis Linked to Otitis, Serotype 9V Infection
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
BOSTON – Otitis on hospital admission and infection with pneumococcal serotype 9V were independently associated with hearing loss in patients who were treated for pneumococcal meningitis in a study presented Sept. 15 at the conference annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Hearing loss is a major cause of morbidity in pneumococcal meningitis, affecting more than 20% of patients with the disease,” study investigator Dr. Sebastiaan G.B. Heckenberg noted. Based on the findings, “patients with coexisting otitis on admission and infection with serotype 9V are at highest risk and should be monitored closely for this outcome,” he said.
Using data from two prospective nationwide cohort studies in the Netherlands, Dr. Heckenberg of the Academic Medical Center in Amsterdam and colleagues identified 531 adults who survived pneumococcal meningitis from 1998-2002 and 2006-2009. All patients underwent neurologic examination at discharge and grading via the Glasgow Outcome Scale (GOS), with an unfavorable outcome defined as a GOS grade of 1-4. Additionally, the majority of patients had audiograms within 1 year after discharge.
Of the 531 patients, 112 experienced “any” hearing loss (defined as audiogram-assessed uni- or bilateral hearing loss of at least 20 decibels within 1 year post discharge, or hearing loss at discharge). A total of 47 experienced severe hearing loss (defined as World Health Organization hearing loss of at least grade 2, or hearing loss as a cause of unfavorable outcome at discharge).
In patients with any hearing loss, otitis on admission was reported in 53%, which was significantly higher than the 32% observed in patients with no hearing loss, Dr. Heckenberg stated. In fact, “on admission, otitis was the only characteristic that was associated with any hearing loss,” he said. “Severity of disease as reflected by low scores on the Glasgow Outcome Scale, systolic blood pressure, and [cerebrospinal fluid] white cell counts were not related to hearing loss in this group.”
With respect to severe hearing loss, otitis on admission was not significantly associated, “but there was a trend among severe hearing loss patients to have lower CSF white cell counts,” Dr. Heckenberg noted. Furthermore, an analysis of hearing loss incidence relative to pneumococcal serotype, which was available for 490 of the 531 patients, showed that serotype 9V was significantly associated with severe hearing loss, he said.
Of interest, Dr. Heckenberg noted, was that severe hearing loss was significantly less common among patients who received dexamethasone. Of the 530 patients for whom the information was available, 10 (4%) of the 240 patients who received dexamathasone experienced significant hearing loss, compared with 37 (13%) of the 290 patients who did not take the glucocorticoid. This finding suggests that hearing loss associated with pneumococcal meningitis could be a function of inflammation, which is mediated by the steroid, he said. Further research into the preventive effect of glucocorticoid therapy is warranted, he noted.
Dr. Heckenberg and his colleagues had no financial disclosures.
Major Finding: Hearing loss frequently complicates pneumococcal meningitis and is associated with coexisting otitis on admission and infection with serotype 9V.
Data Source: Two prospective, nationwide observational cohort studies of adults with community-acquired bacterial meningitis in the Netherlands.
Disclosures: Dr. Heckenberg and his colleagues had no financial disclosures.
Timing, Type of anti-TNFa Therapy Linked to L. Pneumophila
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
Timing, Type of anti-TNFa Therapy Linked to L. Pneumophila
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
BOSTON – The risk of Legionnaires’ disease associated with tumor necrosis factor-alpha antagonist therapy is greatest during the first year of treatment and is significantly higher for patients receiving adalimumab or infliximab, compared with etanercept, according to a study reported Sept. 15 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Using data from the prospective French RATIO (Research Axed on Tolerance of Biotherapies) registry, which was designed to collect information on opportunistic and severe bacterial infections and lymphoma in patients treated with anti–tumor necrosis factor–alpha (anti-TNF-alpha) agents, Dr. Fanny Lanternier of the Necker Hospital for Sick Children in Paris and colleagues conducted an incidence and risk factor study to investigate the relationship between the three drugs included in the registry – adalimumab, infliximab, and etanercept – and Legionella pneumophila infection, which they previously reported in patients receiving anti-TNF–alpha treatment. The researchers used the French population as the reference for the incidence analysis and they conducted a case control study – with four anti-TNF-alpha–treated controls per case – to investigate the risk of newly diagnosed cases of L. pneumophila infection. The mean patient age was 53 years.
From Feb. 1, 2004, to Jan.1, 2007, the RATIO registry received reports of 27 cases of laboratory-confirmed L. pneumophila infection. “The overall annual incidence rate of infection for patients on anti-TNF–alpha therapy, adjusted for age and sex, was 47/100,000 patients per year, which represents a 13-fold increased risk, compared with the reference population,” Dr. Lanternier reported. When evaluated by agent, the standardized incidence risk was significantly higher, at 22.3, for patients taking infliximab or adalimumab – both anti-TNF-alpha monoclonal antibody agents – compared with 3.0 for patients taking etanercept, which is a soluble TNF-alpha receptor therapy, she said at the meeting, sponsored by the American Society for Microbiology.
Similarly, in the case-control analysis, exposure to adalimumab or infliximab vs. etanercept was an independent risk factor for L. pneumophila infection, as was the first year of anti-TNF-alpha treatment, Dr. Lanternier said.
Compared with patients with L. pneumophila infection in the French population, anti-TNF-alpha–treated patients with the infection were younger and had a markedly lower infection-related mortality rate at 3.7% vs. the 10%-20% observed in the population not treated with anti-TNF-alpha drugs, said Dr. Lanternier, attributing the difference to the probability that the immunosuppressed patients are more closely monitored.
In a separate study also presented today, Dr. Alfred F. Sorbello, medical officer for the Food and Drug Administration reported an association between L. pneumophila infection-related mortality and onset of the infection within 90 days of initiating anti-TNF-alpha therapy in patients of younger mean age receiving concomitant steroids or methotrexate. This finding was based on a review of post-marketing adverse event reports for 21 of 80 patients from the FDA Adverse Event Reporting System between 1999 and 2010. Although the results are limited by the small number of patients included in the analysis because of missing data, lack of randomization, and underreporting, they do suggest a key role for TNF-alpha in host defense against L. pneumophila, and they point to the need for clinical vigilance with appropriate diagnostic testing and treatment in these patients, he stressed.
Dr. Lanternier disclosed no conflicts of interests. One of her co-investigators, Dr. Dominique Salmon, disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello had no financial conflicts. The investigators did not give information on who funded the study.
Major Finding: Treatment with some anti–tumor necrosis factor agents increase the risk of L. pneumophila infection as much as 22-fold.
Data Source: Incidence and risk analysis of data from a prospective French database on L. pneumophila associated with tumor necrosis factor–alpha antagonists; Analysis of post-marketing adverse events from FDA Adverse Event Reporting System.
Disclosures: Dr. Lanternier disclosed no conflicts of interests. Co-investigators Dr. Dominique Salmon disclosed research relationships with Schering, Wyeth, and Abbott. Dr. Sorbello disclosed no financial conflicts.
Clostridium difficile Colitis: IV Immunoglobulin Beneficial to Severe, Refractory Patients
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
Clostridium difficile Colitis: IV Immunoglobulin Beneficial to Severe, Refractory Patients
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
BOSTON – Single-dose intravenous immunoglobulin may be an effective adjunctive therapy for patients with severe, recurrent Clostridium difficile colitis that is refractory to standard treatment, according to data presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a retrospective review of 15 patients whose C. difficile colitis was unresponsive to standard therapy with metronidazole, vancomycin, and/or other agents, adjunctive treatment with a single dose of intravenous immunoglobulin (IVIG) led to clinical improvement in 87% of the cases, reported lead investigator Dr. Bienvenido G. Yangco of the Infectious Disease Research Institute in Tampa.
“Thirteen of the 15 patients had improvement or resolution of diarrhea, fever, and leukocytosis and were eventually discharged from the hospital,” he said. “One patient who demonstrated initial improvement was readmitted to the hospital 1 month later for recurrent C. difficile colitis [CDC], while 10 of the remaining 11 patients who had a repeat C. difficile toxin test remained negative after IVIG and have maintained clinical improvement to date.”
The patients included in this review were treated between January 2009 and July 2010 and were C. difficile cytotoxin positive with diarrhea, abdominal pain, distention, fever, leukocytosis, radiographic or colonoscopic evidence of colitis, and persistent or recurrent clinical manifestations despite receiving standard therapies, which included metronidazole (12 patients), vancomycin (13 patients), nitazoxanide (13 patients), and probiotics (9 patients), Dr. Yangco explained. The patients ranged in age from 61 to 92 years, with a mean age of 77, and had the following risk factors for CDC: advanced age, recent antibiotic exposure, and hospitalization, he said.
Of the 15 patients, 2 received a single IVIG dose of 200 mg/kg and 13 received a single 400-mg/kg dose. Among the patients receiving the higher dose, two underwent colectomy. One of these patients, whose colectomy was related to colorectal cancer, had resolution of CDC, and the other patient died, Dr. Yangco said. None of the patients had adverse effects from the IVIG treatment.
The findings are clinically relevant given the dramatic increases in the incidence and severity of C. difficile colitis in recent years and the suboptimal clinical outcomes observed with standard therapies, despite the relative nonexistence of C. difficile resistance to these drugs in vitro, said Dr. Yangco. He attributed the clinical/microbiological discrepancy to “low or waning antitoxin antibody levels in patients with C. difficile colitis.”
Although passive immunotherapy with IVIG in patients who don’t respond to standard treatment has been used since 1991, and its efficacy has been reported in small case series and observational studies, no controlled trials of IVIG in CDC have been performed to date, said Dr. Yangco. “It should be noted that the IVIG doses in the published studies vary from 200 to 1,250 mg/kg for 1-5 consecutive days or once every 3 weeks for 2-3 doses, and in those reports there have been definite benefits and possible efficacy observed with IVIG,” he said. “None of the published studies” has discouraged its use.
Although the findings of the current study are limited by the small number of patients and the retrospective design, the apparent efficacy of single-dose IVIG for the treatment of severe, recurrent CDC “warrants further investigation in prospective, controlled studies,” Dr. Yangco stressed.
“It is very likely that [the] high cost of IVIG – often cited as an obstacle to its use – might be offset in these patients by the benefits associated with sustained clinical improvement, including shorter hospital stays and fewer readmissions.” Furthermore, he said, “changing patterns of C. difficile colitis are compelling reasons for us to reexamine and change our current approach to managing the disease.”
Dr. Yangco disclosed financial relationships with Sanofi Pasteur, Gilead Sciences, Pfizer, Tibotec, Merck, and J.G. Johnson. He noted that this study was not funded by a pharmaceutical company.
Investigational Fidaxomicin Tops Vancomycin for Recurrent C. Difficile Infection
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
From the annual Interscience Conference on Antimicrobial Agents and Chemotherapy
Investigational Fidaxomicin Tops Vancomycin for Recurrent C. Difficile Infection
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
BOSTON – Fidaxomicin is superior to vancomycin for the treatment of recurrent Clostridium difficile infection, investigators reported Sept. 14 at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
In a randomized controlled trial comparing the efficacy of fidaxomicin, a narrow-spectrum macrocyclic antibiotic, and vancomycin in 128 patients with recurrent Clostridium difficile infection (CDI) within 90 days of treatment for the initial CDI episode, fidaxomicin was associated with a 45% reduction in a subsequent CDI recurrence relative to vancomycin. Specifically, 13 of 66 patients (19.7%) randomized to fidaxomicin therapy experienced another recurrence within 4 weeks, compared with 22 of 62 (35.5%) patients randomized to treatment with vancomycin, reported lead investigator Dr. Oliver A. Cornely of the University of Cologne in Germany.
The findings add to the body of evidence supporting the superiority of fidaxomicin over vancomycin for the prevention of CDI recurrences, Dr. Cornely said, alluding to data from two North American and European phase III randomized controlled trials that enrolled a total of 1,164 patients in which he and his colleagues observed a 45%-50% reduction in the recurrence of CDI following an initial recurrence. The current investigation comprised a nested cohort of subjects from these trials who experienced a subsequent recurrence of CDI following treatment, he said.
The patients were at least 16 years old (mean age 63 years) with acute CDI symptoms and a positive stool toxin test. They were randomized to receive 200 mg of fidaxomicin twice daily or 125 mg of vancomycin four times daily, each for 10 days. The study end point was an additional CDI recurrence, defined as diarrhea and a positive stool test within 4 weeks, said Dr. Cornely at the conference, sponsored by the American Society for Microbiology.
Of the 13 recurrences in the fidaxomicin group, 5 occurred within the first 14 days following treatment and 8 occurred between days 15-40. In the vancomycin group, 17 of the recurrences occurred within the first 14 days and 5 occurred between days 15-40, Dr. Cornely reported. The rate of recurrence at 2 weeks was significantly different between the two groups, he said, noting that the later recurrences observed in the fidaxomicin group reflects the likelihood that these recurrences were the result of re-infection from the environment. In contrast, “the earlier recurrences following vancomycin treatment most likely were predominantly due to relapse from persistent spores in the GI tract.”
Because higher age is a known risk factor for recurrent CDI, the investigators separated the patients by age into three groups: 18-54 years; 55-74 years; and 75 years and older, and compared the recurrence rates between the groups. “The risk of second recurrence was 2.7-fold greater among patients 75 years and older, compared with those up to 55 years,” Dr. Cornely said, noting that no differences were observed in the rates of recurrence between patients in the 55-74 years and the 18-54 years groups.
“The efficacy of fidaxomicin at preventing CDI recurrence is likely due to its potent bactericidal activity against C. diff while sparing other protective spores,” Dr. Cornely said. Previous studies have demonstrated that fidaxomicin has a negligible impact on the intestinal flora, while vancomycin causes major perturbations of the anaerobic flora, he explained. “This may explain the differences between the two drugs in the prevention and treatment of recurrent CDI.”
The randomized, controlled phase III trials and the nested cohort study were funded by Optimer Pharmaceuticals, manufacturer of fidaxomicin. The drug is “the first in a new class of antibiotics called macrocycles, which inhibit the bacterial enzyme RNA polymerase, resulting in the death of C. difficile,” according to the drug company.
Dr. Cornely provided no additional disclosures.
From the annual Interscience Conference on Antimicrobial Agents and Chemotherapy
Major Finding: Fidaxomicin was associated with a 45% reduction in the number of repeat C. difficile infections compared to vancomycin following an initial recurrence.
Data Source: Nested cohort of phase III randomized trial comparing the efficacy of fidaxomicin and vancomycin in the prevention of recurrent C. difficile infections.
Disclosures: This study was funded by Optimer Pharmaceuticals, maker of fidaxomicin.