User login
Dexmedetomidine for Sedation in Children
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
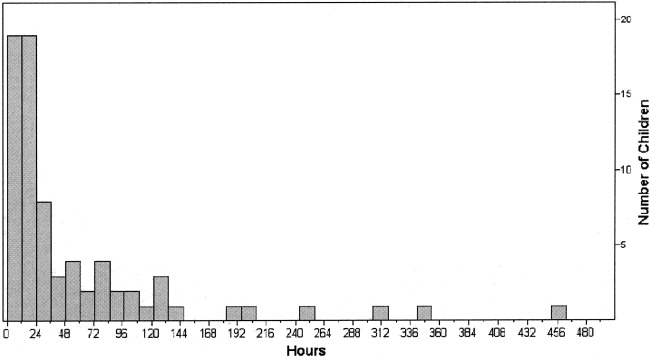
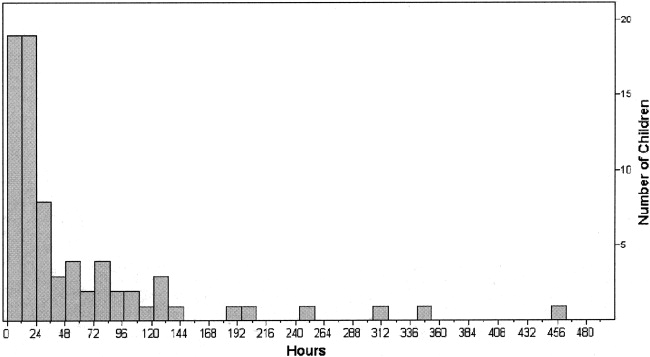
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.
| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
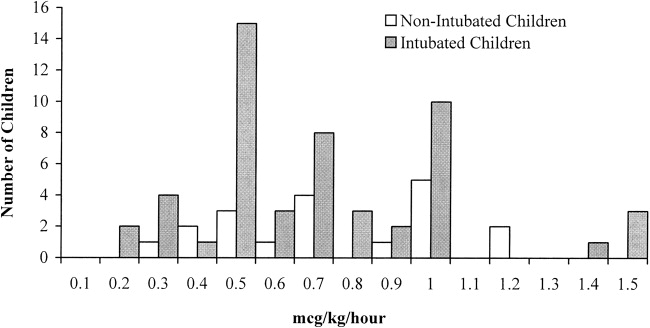
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.


| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
Sedation is commonly administered to hospitalized children.16 An appropriate sedation level is needed to reduce agitation, to facilitate tolerance of invasive therapies, and to prevent invasive devices from being dislodged.16 Age and developmental level can significantly affect the effectiveness of sedation.13 Commonly used medications, such as benzodiazepines and opioids, can adequately sedate children but are difficult to titrate to reach an adequate or consistent level of sedation.13 Sedation of spontaneously breathing children is an even greater challenge because sedation can cause significant and variable respiratory depression and the need for mechanical ventilation.13
Dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL) is a centrally acting 2‐adrenergic receptor agonist that provides a titratable level of sedation with little respiratory depression when delivered by continuous infusion.69 Dexmedetomidine is approved by the U.S. Food and Drug Administration for the short‐term (<24‐hour) sedation of critically ill adults in the ICU setting.47 Despite the potential utility of dexmedetomidine in pediatric critical care, only a few published case series have described its use in children,5, 1020 and no published reviews have examined its use in children for longer than 24 hours. Although the elimination half‐life of a single dose of dexmedetomidine is 3 hours, the duration of action following discontinuation of a continuous infusion in children is also unknown.21 Reported side effects in adults of the use of dexmedetomidine include hypotension and bradycardia, but the safety of prolonged infusions in children has not been reported.
In this study, we describe our experience with the use of dexmedetomidine for sedation of children hospitalized in the pediatric ICU. Dexmedetomidine was administered off‐label for a variety of indications and for durations allowed to exceed 24 hours. Our objective was to retrospectively evaluate the efficacy and complication profile of dexmedetomidine in this population.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at Connecticut Children's Medical Center, and the criteria for informed consent were waived because of its retrospective nature.
Dexmedetomidine was added to the formulary by the Pharmacy and Therapeutics Committee of the study institution in December 2003. Prescribing was restricted to the pediatric intensive care unit (ICU). We retrospectively examined the medical records of all children who received dexmedetomidine for sedation between December 2003 and October 2005. Patients were identified from pharmacy records maintained for quality improvement purposes. The chart abstraction was performed by 2 of the investigators (C.L.C. and D.K.). Audits for uniformity were performed twice during the data abstraction by the principal investigator (C.C.). Dexmedetomidine was administered in all cases without a loading dose. Data were collected regarding hospital course, medications received, amount and duration of dexmedetomidine received, and complications associated with use of dexmedetomidine. During the review period, hemodynamic variables (heart rate, systolic blood pressure, and diastolic blood pressure) were recorded at least hourly for patients receiving dexmedetomidine. Adverse events were defined as occurring during infusion of dexmedetomidine. These events were determined after examination of previous publications describing associated adverse events7, 9, 14 and included abnormalities in hemodynamic parameters (hypotension, hypertension, tachycardia and bradycardia) and respiratory parameters (bradypnea and tachypnea). Values below or above the 5% or 95% normal range for age were considered abnormal. The Pediatric Risk of Mortality (PRISM) III score was used to quantify illness severity on admission to the ICU.22 The effective dose of dexmedetomidine was defined as the dose the patient received for the longest period.
Sedation Regimen at Study Institution
At the study institution, the typical initial therapy for sedation of spontaneously breathing or mechanically ventilated children is a combination of medium‐duration opioids and benzodiazepines, such as morphine and lorazepam. Although sedation scores were not routinely assessed during the study period, the level of sedation was targeted by the nursing staff and the attending physician to maintain comfort, reduce agitation, and allow for tolerance of treatment received. At the study institution these medications are initially administered on an as‐needed basis. If the patient requires additional sedation, they are scheduled every 2 to 4 hours plus given on an as‐needed basis for breakthrough agitation. If additional sedation is still required, the opioid is changed to a continuous infusion of fentanyl, along with scheduled lorazepam, titrated to achieve the desired level of sedation. In patients who require deeper sedation, additional medications such as a barbiturate, ketamine, or chloral hydrate are added.
Statistical Analysis
Clinical characteristics and differences in outcomes were compared using the Student t test for comparison of normally distributed continuous variables, the Mann‐Whitney U test for comparison of continuous variable not normally distributed, the Kruskal‐Wallis test for comparison of continuous variables among more than 2 groups using t tests, and the chi‐square test for comparison of categorical variables. Data was analyzed by case, not by patient, because only a small number of children received dexmedetomidine more than once during the same ICU admission. Most children who received dexmedetomidine more than once received the medication again on subsequent admissions to the ICU. A P value less than 0.05 was considered statistically significant. Data were analyzed using JMP statistical software (version 6.0.2; Cary, NC).
RESULTS
Dexmedetomidine was administered 74 times to 60 children (median age 1.5 years, range 0.117.2 years) during the study period. Most of the patients were male (57%); 53% were white, 23% were Hispanic, 16% were African American, and 8% were designated other. The median PRISM III score was 10 (017). The chronic illness profile and indications for admission are given in Table 1.
| Chronic illness | |
|---|---|
| |
| Congenital heart disease | 30% |
| Chronic respiratory disease (other than asthma) | 24% |
| None | 21% |
| Chronic neurological/developmental delay | 20% |
| Asthma | 11% |
| Other | 14% |
| Indications for ICU admission | |
| Respiratory distress/failure | 43% |
| After corrective cardiac surgery | 19% |
| After other surgery | 18% |
| Asthma exacerbation | 9% |
| Other | 11% |
We found that dexmedetomidine was administered for 3 major indications: (1) as an additive supplementing ongoing sedation judged to be inadequate by the treating physician, (2) in anticipation of extubation to facilitate weaning of other sedation medications, and (3) in spontaneously breathing, nonintubated children to provide a titratable level of sedation without respiratory depression. Children could have more than 1 indication for using dexmedetomidine.
In 36 cases (49%), dexmedetomidine was administered for more than 24 hours. In all children the median effective dose for maintenance of adequate sedation was 0.7 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 23 hours (range 3451 hours; Figs. 1 and 2). Children who received dexmedetomidine for at most 24 hours had a significantly lower effective dose (median 0.5 g/kg per hour, range 0.22.5 g/kg per hour) than did those who received dexmedetomidine for more than 24 hours (median 1 g/kg per hour, range 0.32 g/kg per hour; P = .006). Comparisons of demographics and outcomes based on duration of infusion are given in Table 2.


| Dexmedetomidine received for 24 hours (n = 38) | Dexmedetomidine received for >24 hours (n = 36) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2) | 2.7 (0.415.5) |
| Male sex | 55% | 58% |
| Race/ethnicity | ||
| African American | 16% | 17% |
| White | 53% | 53% |
| Hispanic | 21% | 25% |
| PRISM III score | 10 (017) | 10 (017) |
| Duration of infusion (hours) | 12 (324)* | 73 (27451)* |
| Effective dose (g/kg per hour) | 0.5 (0.22.5)* | 1 (0.32)* |
| ICU length of stay (hours) | 95 (16876)* | 360 (451634)* |
| Incidence of complications | 21% | 19% |
In 53% of cases (n = 39), the dexmedetomidine was used to supplement ongoing sedation that was judged inadequate. In these patients the median effective dose was 0.9 g/kg per hour (range 0.252 g/kg per hour), with a median duration of therapy of 66 hours (range 6451 hours). In this group of patients for whom dexmedetomidine was used to supplement ongoing sedation were 4 patients whose dexmedetomidine was stopped because it was perceived as ineffective by the treating physician. In this subset of patients (n = 4), the median maximal dose was 1.5 g/kg per hour (range 0.81.5 g/kg per hour), and the median duration of infusion was 62 hours (range 1098 hours).
In 41% of cases (n = 30), the dexmedetomidine was used in anticipation of extubation in order to facilitate the weaning off other sedative medications. In these patients, the median effective dose was 0.5 g/kg per hour (range 0.22.5 g/kg per hour), with a median duration of therapy of 14 hours (range 353 hours). A comparison of sedative use before and after dexmedetomidine showed a significant reduction in the use of fentanyl infusions (43% vs. 17%; P = .009) and scheduled lorazepam (30% vs. 10%; P = .02). The median time to extubation after stopping the infusion was 0.6 hours. In 7 children, dexmedetomidine was continued following extubation for a median of 19 hours (range 0.8243.5 hours).
In 26% of cases (n = 19), children were extubated and spontaneously breathing when the dexmedetomidine was initiated. Compared with intubated children, the children who were extubated and spontaneously breathing were significantly older (P = .02) and had a higher level of acute illness at admission, as quantified by the PRISM III score (P = .049). There were no significant differences in sex or race (Table 3). The median effective dose, maximum dose, and duration of dexmedetomidine use did not differ between intubated and nonintubated children (Table 3 and Fig. 2).
| Intubated (n = 55) | Not intubated (n = 19) | |
|---|---|---|
| ||
| Age (years) | 0.9 (0.117.2)* | 4.2 (0.315.5)* |
| Male sex | 58% | 53% |
| Race/ethnicity | ||
| African American | 16% | 16% |
| White | 49% | 63% |
| Hispanic | 24% | 21% |
| PRISM III score | 8 (017)* | 11 (017)* |
| Duration of infusion (hours) | 22 (3451) | 30 (6302) |
| Effective dose (g/kg per hour) | 0.7 (0.22.5) | 0.7 (0.31.2) |
| Maximum dose | 0.7 (0.22.5) | 0.7 (0.31.2) |
In most cases (74%), the dexmedetomidine was stopped because the child no longer required sedation. Other indications for stopping the dexmedetomidine were inadequate level of sedation (7%), need for a longer duration of sedation (16%), and response to an adverse effect (3%).
Most children (80%) experienced no adverse effects during the dexmedetomidine infusion. The most common adverse effects identified were hypotension (9% of all cases), hypertension (8% of all cases), and bradycardia (3% of all cases). Only 1 child developed more than 1 complication (bradycardia and hypertension). In 93% of children who experienced one of these adverse effects (n = 14 of 15), it either resolved without treatment (n = 9) or after withholding or decreasing the dose of dexmedetomidine (n = 5). One child received a fluid bolus for hypotension. The incidence of adverse effects did not differ based on indication for therapy, indication for ICU admission, or chronic disease. Children with cardiac disease or undergoing corrective cardiac surgery also did not have an increased incidence of adverse effects (26% vs. 18%; P = .51). The incidence of adverse effects did not increase with increased duration of therapy (Table 2). A comparison of those who experienced a complication and those who did not showed no differences in the maximal dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) or the effective dose (0.6 0.2 vs. 0.8 0.4 g/kg per minute; P = .1) of dexmedetomidine. In those who experienced a complication, the mean dose of dexmedetomidine administered at the time of the complication was 0.7 0.3 g/kg per minute. When comparing the doses of dexmedetomidine administered at the time of complications, there were no difference in dose based on type of complication. However, patients with bradycardia had a somewhat higher dose (0.9 0.4 vs. 0.6 0.3 g/kg per minute; P = .89) than did patients who experienced other complications, although this was not statistically significant.
DISCUSSION
Dexmedetomidine may have a potentially useful role as a titratable, short‐acting sedative in hospitalized children. However, there are little data regarding pediatric dosage, efficacy, or safety. Off‐label usage of medications is common in pediatrics because of the relatively small number of children admitted to the hospital and the difficulties in performing large clinical trials of children. Clinicians in practice rely on small case series, such as this review, to provide useful information about safety, dosage, and potential duration of therapies. This study was performed in an ICU setting. However, the data can potentially be extrapolated to other hospitalized children.
Several authors have described the effectiveness of dexmedetomidine in children for short‐term or procedural sedation.5, 1016 In a prospective study by Berkenbosch et al.,12 48 children received a dexmedetomidine infusion of 0.51 g/kg per hour for noninvasive procedural sedation. In a retrospective review by Chrysostomou et al.,14 38 children received dexmedetomidine infusions of 0.10.75 g/kg per hour following cardiac or thoracic surgery. In a prospective study by Tobias et al.,5 mechanically ventilated children received a dose of 0.250.5 g/kg per hour for up to 24 hours. Dexmedetomidine was an effective sedative in all these pediatric case series.
In our cohort of children, dexmedetomidine appeared to be effective and to have few adverse effects when administered for durations allowed to exceed 24 hours. The drug's properties make it particularly promising for the maintenance of adequate sedation while weaning patients from mechanical ventilation. Unlike benzodiazepines and opioids, dexmedetomidine causes little respiratory depression and so allows for weaning from mechanical ventilation while simultaneously decreasing the dosage of longer‐acting sedative agents. Dexmedetomidine may also be useful as an additive to supplement ongoing sedation in spontaneously breathing children. This pharmacologic profile makes it an attractive sedative agent in the pediatric ICU setting. In this cohort, only a small number of children experienced adverse effects, none of which were associated with increased duration of therapy. Almost all these adverse effects resolved either spontaneously or by holding/lowering the dose of the infusion.
Previous case series in adults and previous case reports in children have suggested that dexmedetomidine may be used safely for longer than 24 hours.4, 8, 9, 1718 In studies by Shehabi et al. and Dasta et al.,89 a total of 66 adults received dexmedetomidine for median durations of 72 hours (range 35168 hours) and 54 hours (range 25124 hours), respectively. In these studies the number of adverse effects did not increased based on the duration of therapy. In the pediatric population, Hammer et al. reported 4 days of sedation of a child following tracheal reconstruction,18 and Finkel et al. described the prolonged use of dexmedetomidine in 2 children to facilitate weaning from opioids following heart transplantation.17 There were no complications reported in these pediatric case reports.
This is the first case series in children to describe the use of dexmedetomidine for longer than 24 hours. In larger adult studies, hypotension and bradycardia were the most common adverse effects noted with the use of dexmedetomidine.7 In a review of 136 adults by Dasta et al., 23% developed hypotension and 4% developed bradycardia.9 Chrysostomou et al. found that 15% of 33 adults admitted to the ICU following cardiac surgery developed hypotension.14 None of these patients became bradycardic.14 This incidence is similar to that found in our review.
This retrospective review had several limitations. Unfortunately, sedation scores were not routinely used in our institution during the period studied, nor were formal guidelines in place for the titration of sedation. These measures would have allowed us to better quantify effectiveness. In addition, these retrospectively collected data may not have accurately captured the adverse effects associated with dexmedetomidine infusions. The population examined was relatively small. Although there was not an increased incidence of adverse effects in certain subgroups (ie, cardiac), there was not a sufficient number of children in this review to definitively demonstrate safety.
In this cohort of children hospitalized in the ICU, dexmedetomidine appeared to be an effective sedative and to have few adverse effects when administered for relatively long durations. This pharmacologic profile makes it a potentially attractive medication in the hospital setting. Prospective studies are needed to critically examine the use of dexmedetomidine in the pediatric population.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
- ,.Pediatric procedural sedation and analgesia.Pediatr Clin North Am.2006;53:279–292.
- ,.Procedural sedation and analgesia in children.Lancet2006;367:766–80.
- ,.Pediatric sedation: still a hard long way to go.Pediatr Crit Care.2006;7:186–187.
- .Dexmedetomidine in pediatrics: controlled studies needed.Anesth Analg.2004;98:1809–1818.
- ,.Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam.South Med J.2004;97:451–455.
- ,,.Dexmedetomidine.Curr Opin Crit Care.2001;7:221–226.
- ,,,.The role of the α2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit.J Intensive Care Med.2003;18:29–41.
- ,,, et al.Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects.Intensive Care Med.2004;30:2188–2196.
- ,,.Comparing dexmedetomidine prescribing patterns and safety in the naturalistic setting versus published data.Ann Pharmacother.2004;38:1130–1135.
- ,.Initial experience with dexmedetomidine in paediatric‐aged patients.Paediatr Anaesth.2002;12:171–175.
- ,,, et al.Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg.2004;98:60–63.
- ,,.Prospective evaluation of dexmedetomidine for noninvasive procedural sedation in children.Pediatr Crit Care.2005;6:435–439.
- ,,, et al.Dexmedetomidine for pediatric sedation for computed tomography imaging studies.Anesth Analg.2006;103:57–62.
- ,,, et al.Use of dexmedetomidine in children after cardiac and thoracic surgery.Pediatr Crit Care.2006;7:126–131.
- ,,.The use of dexmedetomidine in pediatric cardiac surgery.Anesth Analg.2006;103:52–56.
- ,.Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation.Anesth Analg.2006;103:68–69.
- ,.The use of dexmedetomidine to facilitate opioid and benzodiazepine detoxification in an infant.Anesth Analg.2004;98:1658–1659.
- ,,, et al.Prolonged infusion of dexmedetomidine for sedation following tracheal resection.Paediatr Anaesth.2005;15:616–620.
- ,.Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin.Pediatr Crit Care.2003;4:203–205.
- ,,.Additional experience with dexmedetomidine in pediatric patients.South Med J.2003;96:871–875.
- ,,.Pharmacokinetics and pharmacodynamics of transdermal dexmedetomidine.Eur J Clin Pharmacol.1994;46:345–349.
- ,,.PRISM III: an updated Pediatric Risk of Mortality score.Crit Care Med.1996;24:743–752.
Copyright © 2008 Society of Hospital Medicine