User login
Epistaxis: A guide to assessment and management
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
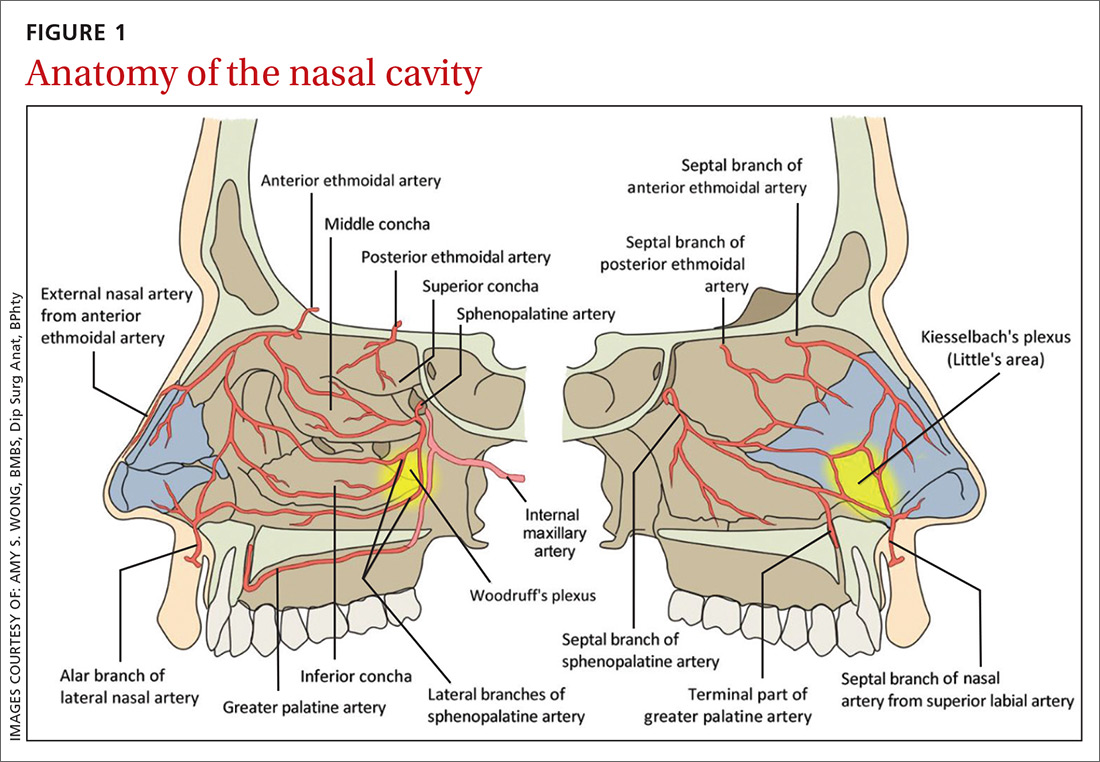
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).
Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
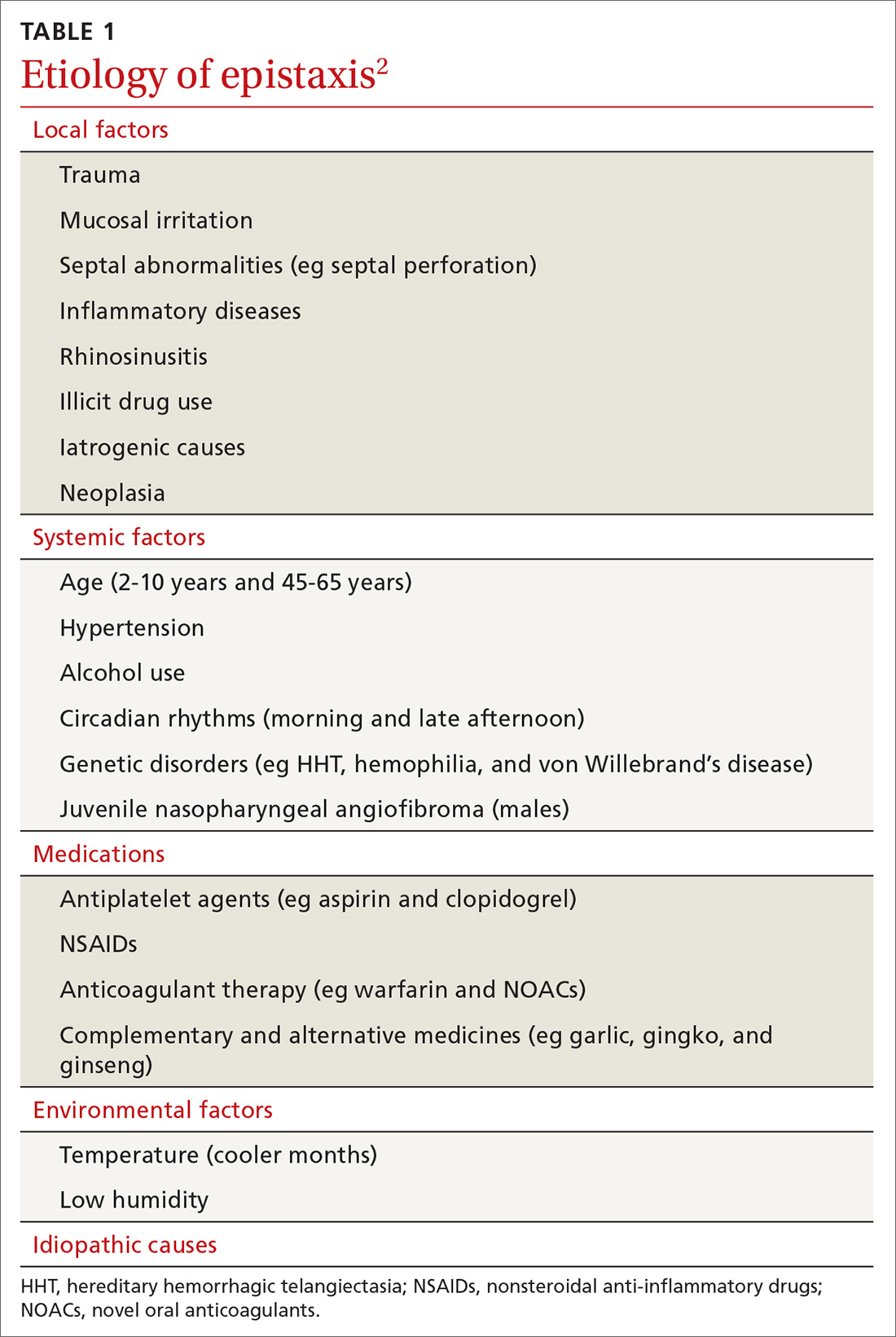
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.
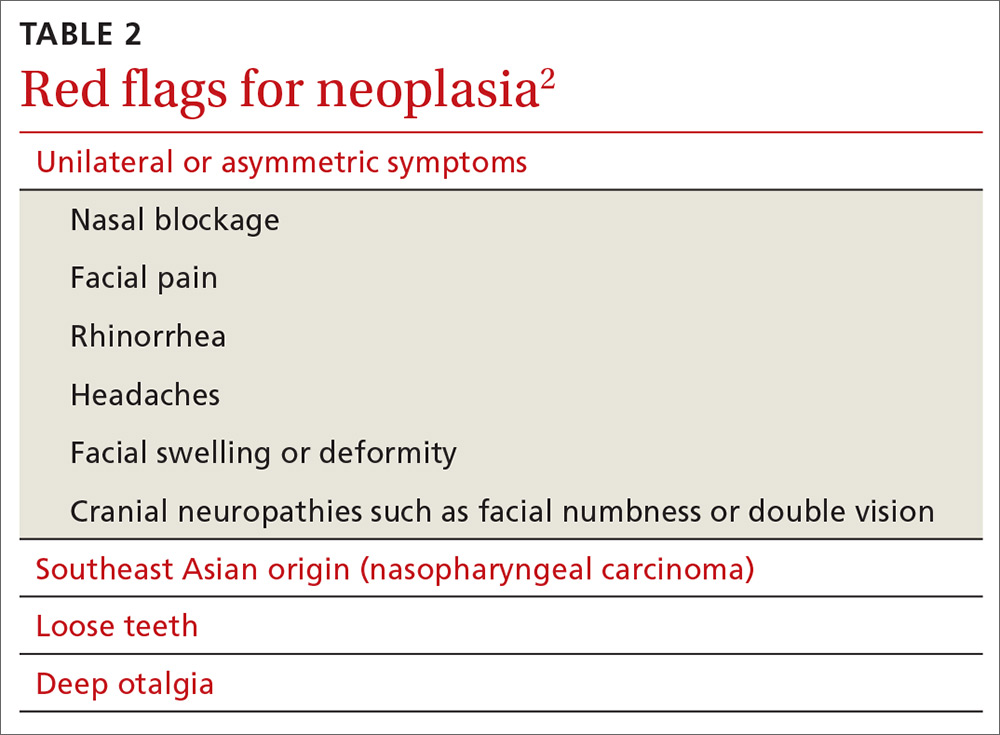
Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.
Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.
Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
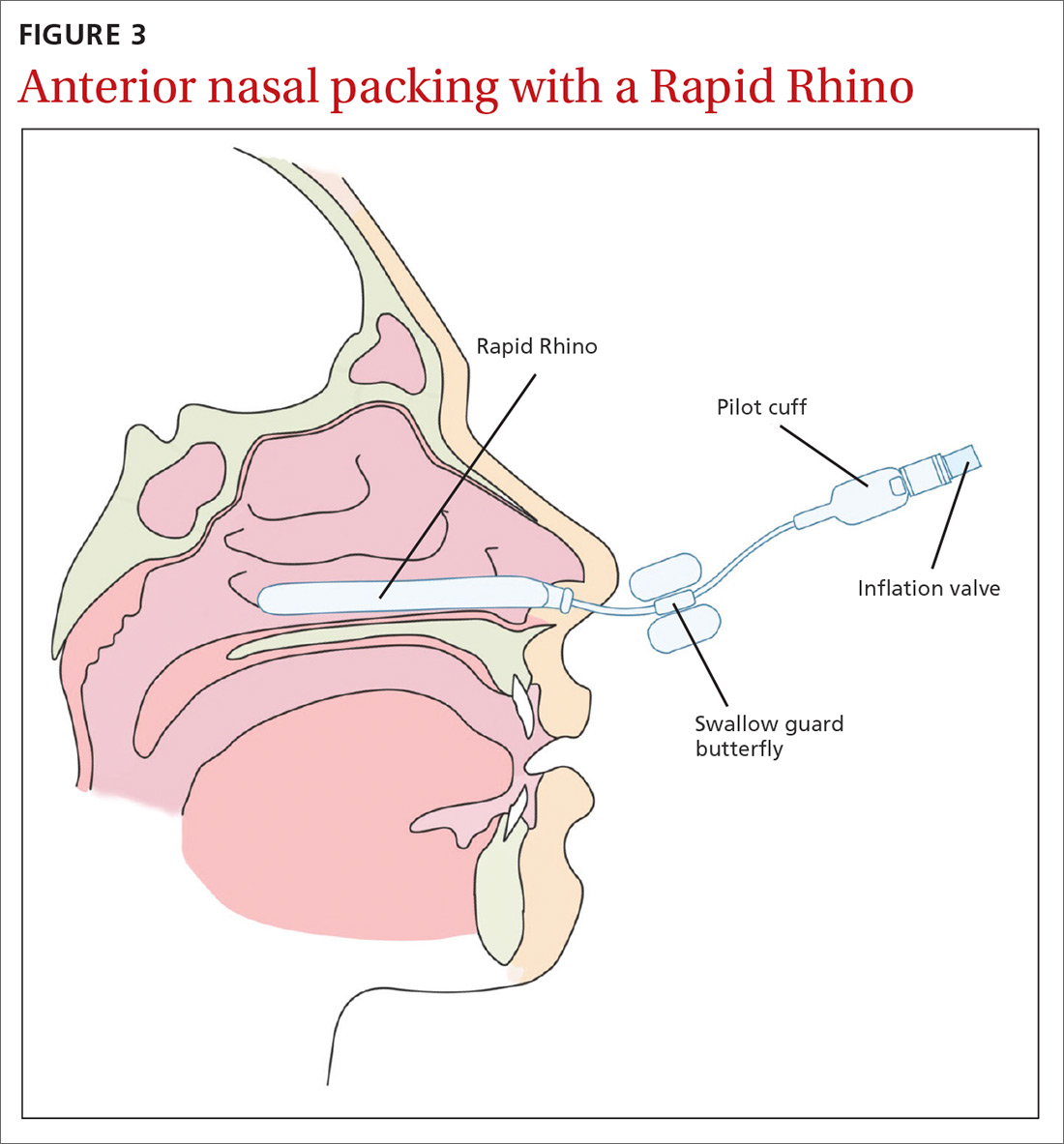
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13
Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
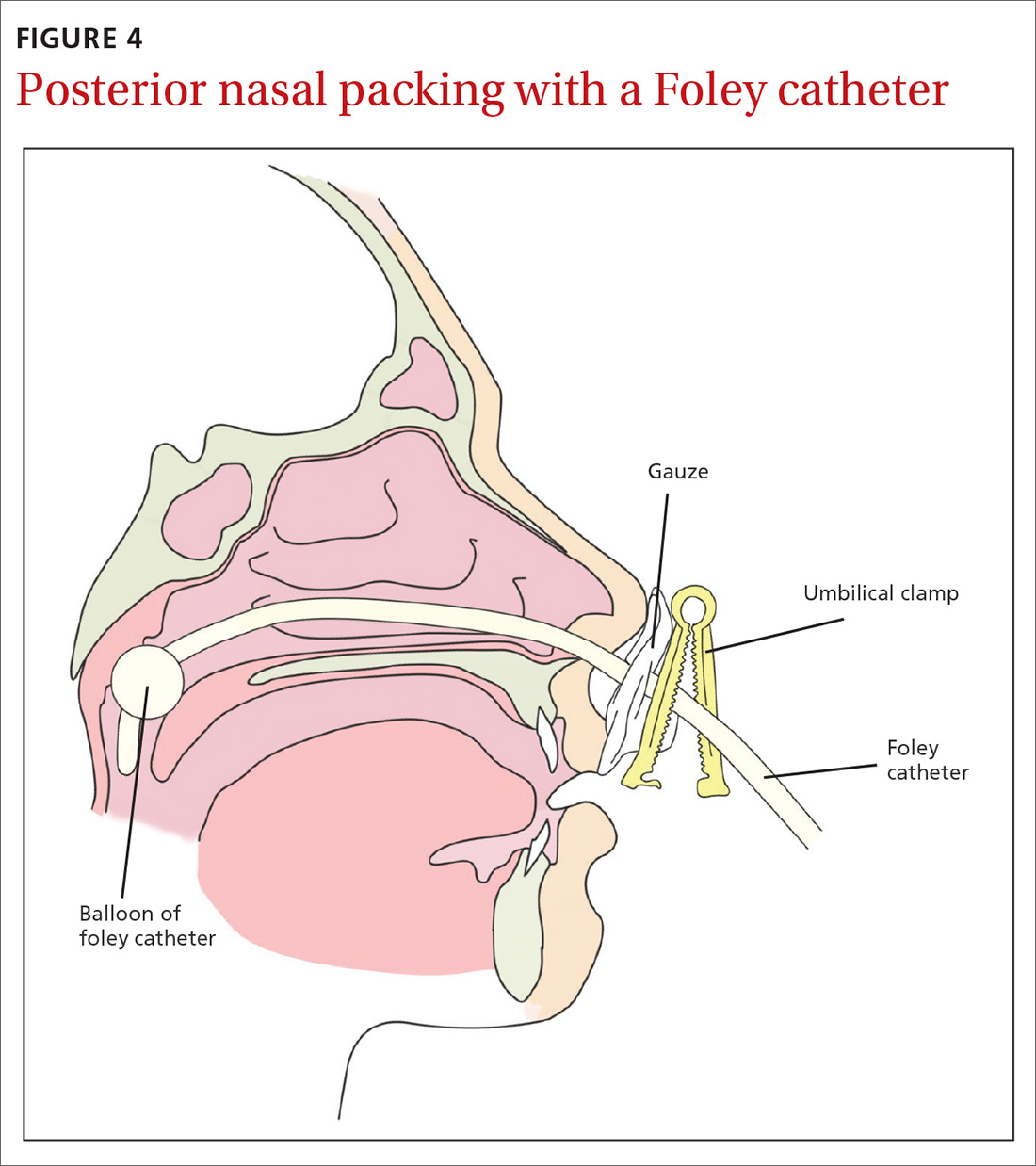
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.
Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6
Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, amy.wong@monashhealth.org.au.
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).
Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.
Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.
Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.
Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13
Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.
Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
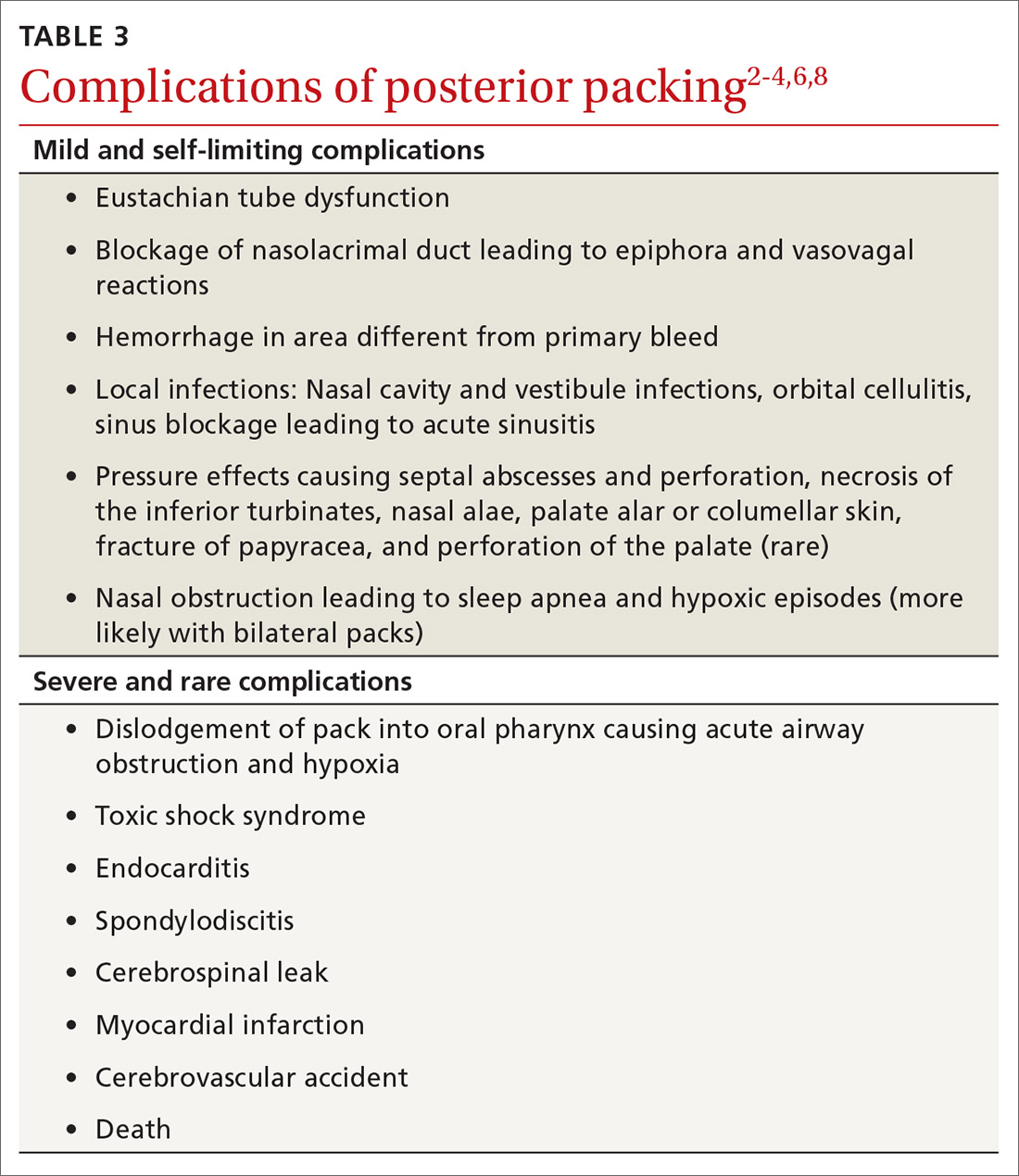
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6
Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, amy.wong@monashhealth.org.au.
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).
Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.
Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.
Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.
Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13
Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.
Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6
Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, amy.wong@monashhealth.org.au.
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
From The Journal of Family Practice | 2018;67(12):E13-E20.
PRACTICE RECOMMENDATIONS
› Use topical vasoconstrictor and local anesthetic agents as a first line therapy for epistaxis. Consider the additional use of topical tranexamic acid. A
› Perform chemical cautery with silver nitrate in cases of anterior epistaxis. This approach is cheap, easy to perform, and silver nitrate is readily available. A
› Consider endoscopic sphenopalatine artery ligation in the acute management of posterior epistaxis. It is superior to posterior nasal packing and embolization when it comes to pain, cost-effectiveness, risk, and overall control of bleeding. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series