User login
Trial of Safety Nets in Hospitalized Patients
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
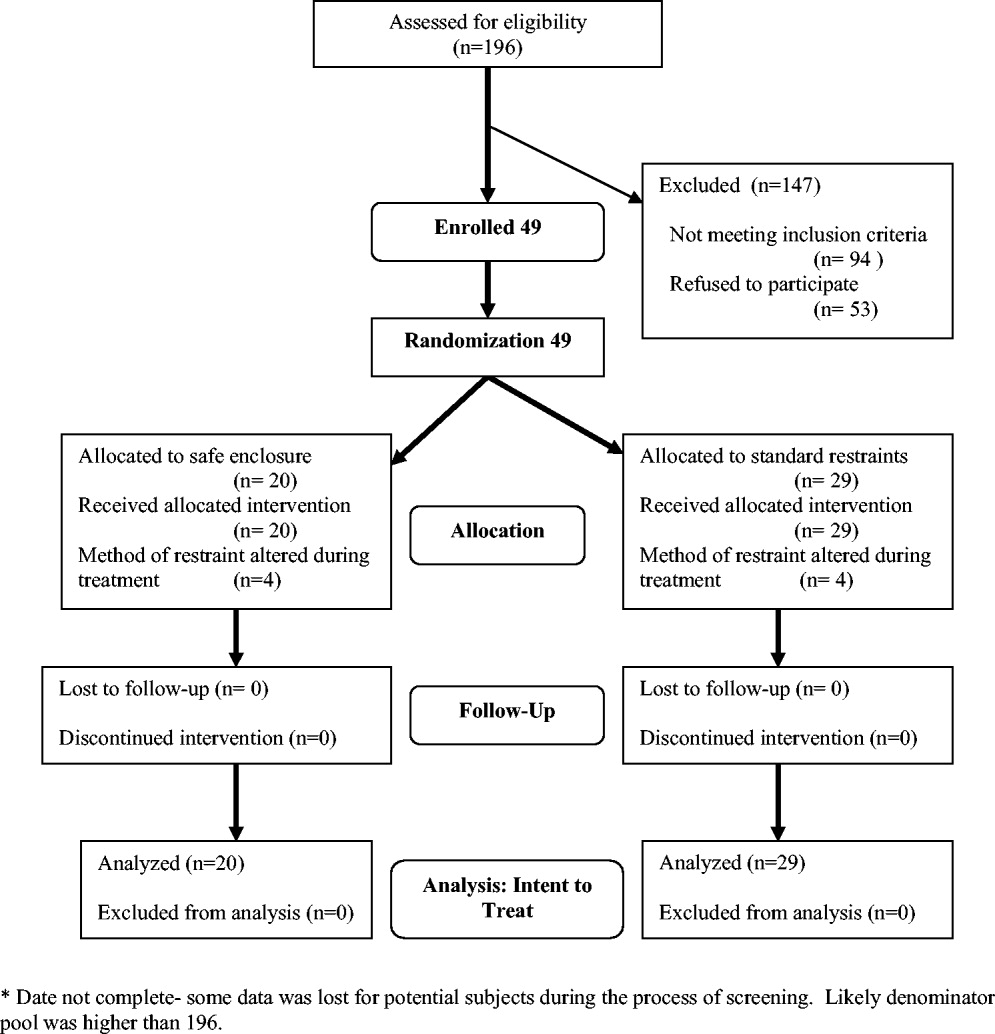
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.
A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
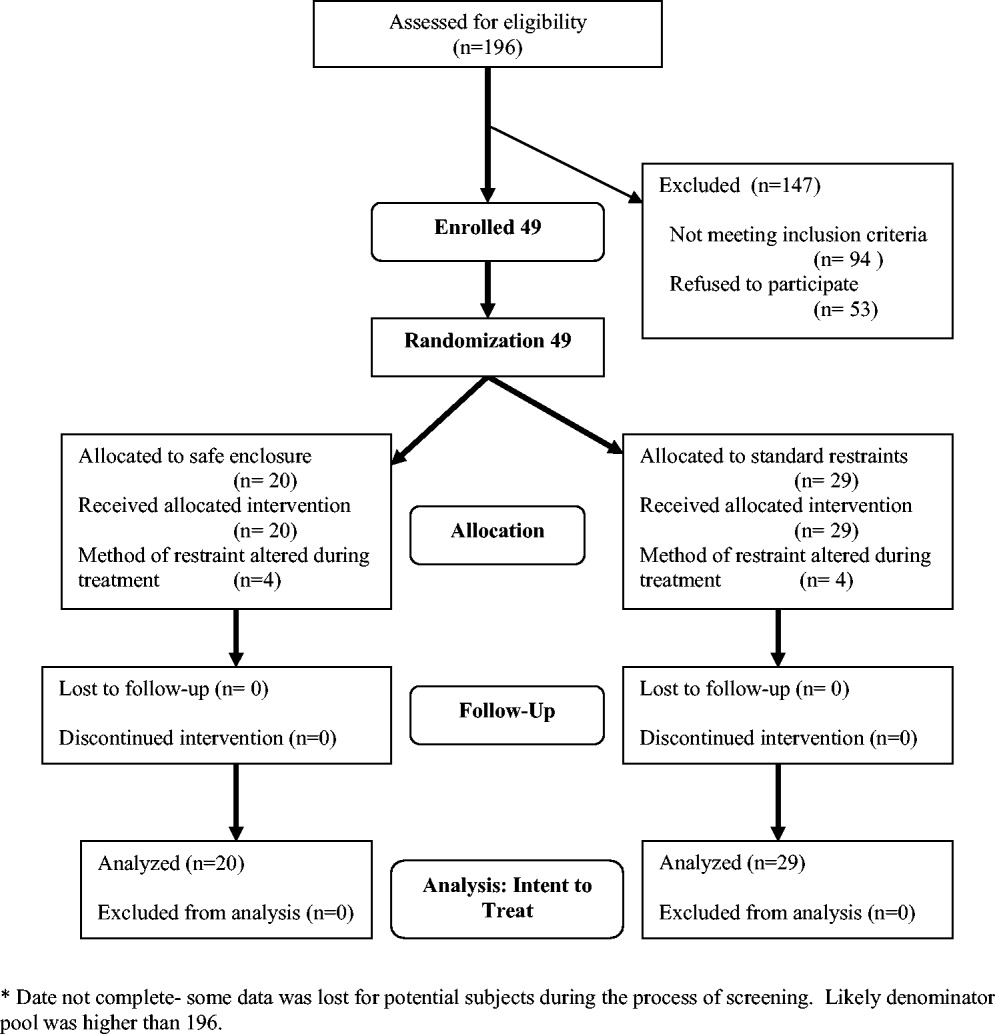
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.

A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
Physical restraints, such as bed rails, Posey vests, and 2‐point and 4‐point soft or hard restraints, are commonly used in acute care hospitals to protect agitated patients from harming themselves or others.1 Yet restraints are viewed by patient advocates and health care practitioners as inhumane and overly restrictive. Furthermore, currently used physical restraints have been linked to minor injuries such as sores and abrasions, intensification of agitation, and even death.2, 3 Hospitals and nursing homes are therefore required to try alternative and less severe means of alleviating agitation and delirium among patients before resorting to physical restraints. However, despite a general dislike of restraints and stricter federal guidelines governing their use, the application of restraints is often unavoidable for some patients. It is estimated that between 4% and 25% of in‐patients will have physical restraints applied at some point during their hospital stay.4
Given these numbers, it is surprising that newer and potentially safer restraint systems have not been explored. Safe enclosures may provide health care facilities with an alternative option. This type of restraint consists of a nylon net canopy that safely surrounds both the patient and the mattress. The potential for safe enclosures to provide a safe, humane, and acceptable method of restraint for both hospital staff and patients warranted investigation. In addition, because this system does not restrict a person's ability to move within the enclosure, the many potential hazards of immobility associated with standard restraints may be reduced or eliminated. However, to our knowledge, there have been no reports published of randomized trials comparing standard restraints to newer and possibly safer restraint systems.
We report a randomized controlled trial that compared the use of safe enclosures with standard restraints among agitated, hospitalized patients. Compared with patients in standard restraints, we hypothesized that safe enclosures would: (1) be perceived as more acceptable and humane by family members, physicians, and nurses; (2) lead to improved health outcomes such as decreased duration of restraint use, decreased agitation, shorter length of stay, decreased need to administer medication to treat agitation, and fewer injuries to the patient.
METHODS
Design and Setting
This was a prospective, single‐center, randomized, controlled trial conducted at a community hospital in Connecticut.
Subjects
Male and female hospitalized patients at least 18 years old in the general medicine in‐patient services at a community hospital in Connecticut were assessed for eligibility to participate in this study if they had been put in restraints by the health care team independent of the study for one of these acute conditions: (1) delirium from any cause, including drug or alcohol withdrawal, or other medical conditions resulting in acute delirium; (2) confusional state from any cause; (3) agitation and disruptive behavior requiring restraints; (4) psychosis, hallucinations, or delusions requiring acute intervention (such as medication, restraints, or sitter); or (5) suicidality. Once in restraints, patients were screened for eligibility to participate in this study.
Exclusion criteria included: (1) need for acute respiratory or hemodynamic support or cardiac or septic shock; (2) terminal illness; (3) documented history of claustrophobia; (4) refusal by the family to give consent; (5) hospital stay < 24 hours; and (6) need for intravenous vasopressors, intubation, or ventilatory support. We also excluded patients who had been in restraints for more than 48 hours prior to potential study enrollment. Because safe enclosure would be a redundant system of restraint for patients requiring more than 1 limb in restraints, those patients were also excluded from the study. Figure 1 shows participation flow.

A number of screened patients were excluded for not being appropriate candidates for the safe enclosure. Of these, 20% required more than 1 limb in restraints, 13% required restraint not for agitation but for IV or catheter protection only, 10% were in critical care or on ventilators, and 13% were not appropriate for various other reasons including claustrophobia. The remaining excluded patients may have been eligible but either were preparing for discharge on screening (26%), were in restraints for more than 48 hours on screening (11%), stayed in the hospital less than 24 hours (4%), or had previously been a study participant (3%).
A stratified permuted block randomization was used to control for age (65 vs. >65 years) and sex (male vs. female) to ensure equal representation in both study arms. The study was approved by the institutional review board of the study site, and written informed consent was obtained by the study coordinator from patients' families. Because eligible patients suffered from acute delirium or agitation, most were not sufficiently cognizant to participate in the consent process. As a result, consent was largely obtained from patients' family members. Although the intent of this trial was to recruit 60 patients over an 18‐month period, the study was closed at 49 after 2 years because of slow recruitment and a lack of remaining funds.
Intervention
The safe enclosure, also known as a net bed or safety net, is an alternative to standard restraints. It consists of: (1) a metal frame that sits on the floor completely enclosing a standard hospital bed and (2) a nylon net canopy that encloses the patient and the mattress. We used the SOMA Safe Enclosure (Vivax Medical Corporation, Torrington, CT,
Procedures
Patients were enrolled in this study from April 2003 to February 2005. Once a patient had been placed in restraints by a physician, the nurse in charge alerted the study investigators by beeper of a potential subject, who was then screened based on the above eligibility criteria. We also actively screened restraint log sheets maintained by the nursing staff on most weekdays to monitor new patients who may have been put on restraints. Subjects were randomized to remain in standard restraints or be transferred to the safe enclosure. The randomization scheme was generated using software available at
Modification of the use of restraints is common in hospital settings, as patient needs fluctuate. In the 2 study groups, restraint crossover did occur at the discretion of the attending physician or nurse. Four patients in the safe enclosure group required either additional restraints or had the safe enclosure removed and alternate restraints applied. Similarly, 4 patients in the standard restraint group were either given the safe enclosure or had additional standard restraints applied. Under the principle of intent to treat, patients remained in their original randomized group for the purpose of analyses. This approach provided the most conservative analyses by keeping the sickest patients in the intervention group, thus guarding against type I error.
Measurements
Baseline data obtained at enrollment included: (1) demographic information, (2) clinical information, and (3) restraint information (ie, time of restraint application, clinical indication for use, type of restraint used, ordering physician, and alternative treatment tried before decision to start restraints).
Primary outcomes included: (1) perception survey scores of family members, physicians, and nurses regarding patient comfort, acceptability, and safety of the restraint device; and (2) patient agitation scores. A preplanned subgroup analysis separated nurses into 2 categories (primary and secondary). The admitting nurse was designated the primary nurse; all other nurses were considered secondary nurses. This analysis was performed to examine any differences in perception resulting from a nurse's level of patient involvement.
Family and provider perceptions were assessed with a self‐administered survey containing 11 items each measured on a 10‐point scale (from 1 = viewed negatively to 10 = viewed positively; maximum score 110 points). Surveyed physicians, nurses, and relatives were asked to rate: (1) patient comfort, (2) accessibility to patient, (3) ease of communication with patient, (4) how calm patient was, (5) perceived safety of patient, (6) patient's feeding convenience, (7) ease of bedpan use, (8) impact on recovery time; (9) how humane and ethical the restraint was, (10) recommendation for use on other patients, and (11) how demanding or difficult caring for the patient was.
Agitation was measured using 2 distinct methods: the Alcohol Withdrawal Assessment Form (AWAF)5 and the Agitated Behavior Scale (ABS).6, 7 Both techniques have been widely used to assess delirium of hospitalized patients. The AWAF measures agitation by analyzing key physiologic indicators such as blood pressure and heart rate on a 0‐ to 22‐point scale. The ABS is a 14‐item scale that measures specific behaviors related to agitation (eg, distractibility, uncooperativeness, and restlessness). Each behavior is rated on a 4‐point Likert scale (0‐3); total score ranges from 0 to 42. Each scale was completed once per 8‐hour shift by the nurse on duty.
Secondary outcomes consisted of total length of stay, duration of restraint use, time from application of restraints until time of discharge, and time from admission until time of application of restraints. Length of stay was calculated as the number of days from the time the patient was admitted to the time the patient was discharged. Time from admission until time of restraint application, duration of restraint use, and time from restraint application until discharge were assessed in minutes. These measurements were based on written restraint order forms and nursing progress reports. Hospital protocol regarding restraint requires hospital staff members to document the application, removal, and adjustment of restraints.
Additional outcomes measured included total amount of medications used to treat agitation and number of injuries incurred. Total amount of medication administered was determined with an equivalence system for different drugs used to treat agitation or delirium.811 Medications were separated into 4 groups: antianxiety medications, antidepressants, antipsychotics, and opioid analgesics. Total amount included both regularly administered and as‐needed dosages of medication. We identified injuries through reports of a subject's primary nurse and by review of medical records.
Data Analyses
Sample size was calculated using a 2‐sample t test formula based on the primary outcome. The study was designed to detect an absolute difference in points of 10% (total absolute score difference of 11 per survey or a total difference of 33). The 2‐sided alpha was initially set at 0.05 and the power at 80%, with an estimated standard deviation of 20. The alpha level was Bonferroni‐adjusted for up to 6 additional comparisons, with each significance level of 0.0071 (z = 2.70).
To assess differences in patient characteristics between the standard restraints and safe enclosure groups, we used the Student t test for continuous variables and Fisher's 2‐sided exact test for categorical variables. Differences in family and staff perceptions of the restraint mechanisms were measured using the Student t test with Satterthwaite's method for calculating variance. However, to account for questions marked not applicable by the responder, weighted scores, defined as the total score divided by the percentage of questions answered, were calculated.
Differences in agitation scores (ABS and AWAF) were analyzed using 2 strategies. First, the ABS and AWAF scores 24 hours after study enrollment were compared across groups using the Student t test with Satterthwaite's method for calculating variance. Then, separate comparisons of the ABS and AWAF scores 48 and 72 hours after enrollment were conducted using the Student t test with a pooled variance. Next, 2 longitudinal analyses were performed using a mixed‐effects (fixed and random) model. These analyses modeled change in the ABS or AWAF scores over (1) the first 3 days and (2) the first 6 days of hospitalization as a function of being restrained with the safe enclosure or being restrained with the hospital's standard restraint systems. For these comparisons, the model included not only the main effects of type of restraint and time, but also the interaction between type of restraint and time and the covariates sex, age, and initial ABS or AWAF score. For these models, a backward elimination procedure was undertaken using a significance level of = 0.05 in order to determine the most parsimonious model.
To determine if the total length of subject stay in the hospital was different between groups, the Student t test was used with Satterthwaite's method for calculating variance. Differences in time from admission until time of restraint application, duration of restraint use, and time from application of restraints until time of discharge were analyzed with the Student t test with pooled variances.
To compare the amount of medication used, equivalent dosage conversions were used for each of the 4 medication categories (antianxiety medications, antidepressants, antipsychotics, and opioid analgesics). To determine if the amounts of these 4 categories of medications differed between groups, the Student t test was used. Last, to determine if there was a difference in the number of patient injuries between groups, Fisher's 2‐sided exact test was used.
RESULTS
Study Population
Of the 49 subjects enrolled in the study, 20 were randomized to the safe enclosure and 29 to standard restraints. This imbalance was likely a result of the premature termination of the study, which in turn was a result of slow recruitment. Table 1 shows selected baseline characteristics of the enrolled subjects. There were no significant differences between the 2 groups in sex, age, patient diagnoses, reason for restraint, or type of medication. However, the subjects randomized to the safe enclosure were less likely to have hypertension than those randomized to standard restraints (36.8% vs. 72.4%, P = .019).
| Variable | All (n = 49) | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) |
|---|---|---|---|
| |||
| Sex (male) | 26 (53.1%) | 11 (55.0%) | 15 (51.7%) |
| Age (years) | 81.3 (13.1%) | 77.2 (15.6%) | 84.2 (10.3%) |
| Alzheimer's disease | 23 (47.9%) | 11 (57.9%) | 12 (41.4%) |
| Dementia | 3 (6.3%) | 2 (10.5%) | 1 (3.5%) |
| Coronary artery disease | 19 (39.6%) | 10 (52.6%) | 9 (31.0%) |
| Hypertension* | 28 (58.3%) | 7 (36.8%) | 21 (72.4%) |
| Congestive heart failure | 6 (12.5%) | 3 (15.8%) | 3 (10.3%) |
| Atrial fibrillation | 7 (14.6%) | 1 (5.3%) | 6 (20.7%) |
| Transient ischemic attacks/cerebral vascular accidents | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Chronic obstructive pulmonary disease | 3 (6.3%) | 1 (5.3%) | 2 (6.9%) |
| Diabetes mellitus | 11 (22.9%) | 5 (26.3%) | 6 (20.7%) |
| Alcohol abuse | 7 (14.6%) | 2 (10.5%) | 5 (17.2%) |
| Drug abuse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Where admitted | |||
| General medicine floor | 41 (83.7%) | 17 (85.0%) | 24 (82.8%) |
| Telemetry | 7 (14.3%) | 2 (10.0%) | 5 (17.2%) |
| ICU | 1 (2.0%) | 1 (5.0%) | 0 (0.0%) |
Primary Outcomes
The rates of response to the perception survey were: relatives/next of kin, 90%; physicians, 90%; primary nurses, 100%; and secondary nurses, 78%. Family members and physicians viewed the safe enclosure significantly more positively than they viewed standard restraints (P < .0001 and P < .0001, respectively; Table 2). There was a trend toward more positive perceptions of the safe enclosure among nurses; however, this trend did not achieve statistical significance (P = .0836). The subgroup analysis of nurses (primary vs. secondary) revealed that secondary nurses viewed the safe enclosure more positively (P = .023). Primary nurses tended to view the safe enclosure more positively than the standard restraints, but the association was not significant (P = .1313).
| Variable | SOMA Safe Enclosure (n = 20) | Standard restraint system (n = 29) | P value (observed power)* |
|---|---|---|---|
| |||
| 1. Perception Survey | |||
| Relative or next of kin | 86.84 | 68.47 | < .0001 (96%) |
| Physician | 83.38 | 65.76 | < .0001 (96%) |
| All nurses | 75.20 | 69.45 | .086 (40%) |
| Primary nurse | 75.45 | 69.72 | .1313 (31%) |
| Secondary nurse | 80.35 | 69.82 | .0230 (58%) |
| 2. Alcohol Withdrawal Assessment Form | |||
| 24 hours | 3.06 | 3.25 | .7972 (6%) |
| 48 hours | 3.23 | 3.40 | .8516 (5%) |
| 72 hours | 3.44 | 2.67 | .6163 (7%) |
| 3. Agitated Behavior Scale score | |||
| 24 hours | 11.93 | 8.33 | .2312 (27%) |
| 48 hours | 6.00 | 8.75 | .3743 (13%) |
| 72 hours | 7.83 | 7.11 | .7762 (6%) |
There were no statistically significant differences between the 2 randomized groups in ABS or AWAF scores 24, 48, or 72 hours after restraint application (Table 2). In addition, there were no statistically significant differences during the study between the groups in the rates of change in ABS or AWAF score . This was the case when looking at the first 3 days of hospitalization as well as the first 6 days (data not shown). All results were also calculated after adjusting for length of stay; this covariate did not affect any of the results.
Table 3 details the results for each perception survey question. Perceived comfort, calmness, and safety of patients were rated higher in the safe enclosure group by physicians, relatives, and all nurses. With the exception of perceived accessibility to patients, relatives rated the safe enclosure higher than standard restraints on all other perception measures. Table 4 illustrates the differences in the responses of primary and secondary nurse to each perception survey question. Primary and secondary nurses viewed the safety of the safe enclosure significantly more positively than they did the standard restraints.
| Variable | Relative/next of kin (n = 16, 28) | Primary and secondary nurses (n = 29, 29) | Physician (n = 29, 29) |
|---|---|---|---|
| |||
| Comfort | (8.78, 7.29).0033 | (7.98, 6.78).0194 | (8.40, 6.77).0003 |
| Accessibility | (8.28, 8.07).6486 | (7.68, 8.29).2236 | (8.35, 7.58).1056 |
| Communication | (9.11, 8.19).0214 | (8.29, 8.12).7333 | (8.40, 8.31).8469 |
| Calmness | (8.72, 6.29).0005 | (7.68, 6.53).0382 | (7.70, 5.92).0062 |
| Safety | (9.11, 6.74) < 0.001 | (8.53, 6.76).0024 | (8.60, 5.96).0002 |
| Feeding convenience | (8.50, 7.04).0164 | (7.11, 7.74).2327 | (8.25, 6.28).0047 |
| Ease of bedpan use | (7.91, 6.06).0224 | (7.36, 6.90).3977 | (6.82, 6.25).5376 |
| Impact on recovery time | (7.53, 6.07).0244 | (6.29, 5.66).3864 | (6.95, 6.43).4254 |
| Humane/ethical | (7.94, 5.50).0026 | (6.88, 6.31).4049 | (7.95, 5.96).0052 |
| Recommend for other patients | (8.71, 5.50).0002 | (7.15, 6.12).1395 | (8.05, 6.04).0037 |
| Ease of caring for patient | (8.44, 5.70) < .001 | (7.55, 6.38).0749 | (8.05, 6.25).0028 |
| Variable | Primary Nurse (n = 20, 29) (SOMA safe enclosure, control)* P value | Secondary Nurse (n = 12, 26) (SOMA safe enclosure, control)* P value |
|---|---|---|
| ||
| Comfort | (7.85, 6.62).0270 | (8.33, 6.81).0346 |
| Accessibility | (7.55, 8.21).2656 | (8.17, 8.16).9902 |
| Communication | (8.21, 8.34).8093 | (8.83, 7.88).0705 |
| Calmness | (7.70, 6.28).0153 | (7.83, 6.85).2001 |
| Safety | (8.55, 6.34).0012 | (8.67, 6.76).0435 |
| Feeding convenience | (7.37, 7.93).3861 | (7.60, 7.42).8282 |
| Ease of bedpan use | (7.53, 6.95).3945 | (7.00, 6.84).8602 |
| Impact on recovery time | (6.16, 5.39).3251 | (7.50, 6.50).2340 |
| Humane/ethical | (6.45, 6.66).7871 | (7.73, 5.96).0571 |
| Recommend for other patients | (6.70, 6.11).4553 | (8.42, 5.92).0075 |
| Ease of caring for patient | (7.65, 6.41).0860 | (7.83, 6.38).0565 |
Secondary Outcomes
There was a trend toward shorter total length of stay, time from admission until restraint application, duration of restraint use, and time from restraint application until discharge among subjects restrained by the safe enclosure compared with those restrained with standard restraints. However, these unadjusted differences were not statistically significant. We examined secondary outcomes after adjusting for 2 covariates, age and sex. Age but not sex affected the results. We found that subjects in the intervention group younger than 80 years of age had a shorter length of stay for 2 of the 4 related outcomes: time of admittance to time of discharge (P = .0199) and time of restraint to time of discharge (P = .0274). Time of admission to time of restraint application and duration of restraint did not differ between groups. The former outcome was not expected to differ between groups.
Additional Outcomes
There were no differences between groups in the amounts of 3 of the 4 types of medications used to treat agitation or delirium (ie, antianxiety medications, antipsychotic medications, opioid analgesic medications). The proportion of patients on these medications did not differ by group (P = .59). Only 5% of patients in standard restraints were on antidepressants, and about 5% were on opioids in each group. There was only 1 minor patient injury recorded during the study. This minor abrasion was to a patient assigned to the standard restraint group. No injuries were reported in the safe enclosure group.
DISCUSSION
We have demonstrated that the SOMA Safe Enclosure may be a more acceptable alternative to the restraints currently in use. Our results show that the safe enclosure was rated as more acceptable by family members, physicians, and secondary nurses in our composite perception scores. The results from the primary nurses did not show a significant difference between the 2 groups. An analysis of the individual perception variables found that family members viewed the safe enclosure as more acceptable for 10 of the 11 variables examined. Furthermore, in this small‐scale study, safe enclosures appeared to be safe, as there were no injuries reported in the intervention group. As stated above, there was 1 minor injury reported in the standard restraint groups.
Restraints are commonly used to protect agitated hospitalized patients from harming themselves or others. Despite the significant reluctance of hospital staff members to use restraints, they continue to be necessary in certain situations. Factors such as a general nursing shortage and the expense required to allocate nursing or other ancillary health care workers as sitters contribute to the use of restraints. Therefore, it is reasonable to conclude that restraint use in some form or fashion will continue into the foreseeable future. There are no clear estimates of the prevalence of restraint use in acute care hospitals. A chart review study from Canada reported physical restraints in about 7.7% of in‐patients.12 Other studies have reported the use of restraints on patients in the range of 4%‐25%.2 Given the prevalence of restraint use in acute care hospitals, surprisingly little innovative research has been undertaken to develop more effective and humane systems of restraint. Furthermore, no research has examined how restraint use may affect important clinical outcomes such as length of stay. To our knowledge, this is the first clinical trial to compare currently used restraints to a newer method of restraint using the SOMA Safe Enclosure.
The idea that restraint use can lead to further agitation is not supported by our data. We observed a decrease in agitated behavior scale scores from 9.6 to 7.4 from the 24‐ and to the 72‐hour assessments; however, these results were not significant and appeared to be more dramatic for the safe enclosure group because of higher baseline levels. Our adjusted analyses of length of stayrelated outcomes indicated an association with age. Total length of stay and time from restraint application until discharge were significantly reduced for those subjects younger than 80 years of age in the safe enclosure group. The basis for this finding is not entirely clear. It may be a chance finding, or there may have been a complex combination of factors at work.
There was a reduction in overall length of stay by 1.5 days among those in the safe enclosure group when compared with the standard restraint group. Similarly, total duration of restraint use of the safe enclosure group was 551 minutes (9 hours) shorter. Although these findings were not significant, they warrant further investigation in a larger trial. If safe enclosure use truly reduces length of stay and duration of restraint use, it is an important finding, for it could translate into meaningful cost savings for acute care hospitals. It is possible, however, that any potential cost savings could be tempered by the additional time required to set up the enclosure. Ethically, if restraints are to be used, their use should be minimized, and in that sense, safe enclosures may help acute care hospitals achieve this goal more effectively.
Limitations of this trial include its small sample size and inadequate power to determine certain outcomes. Although we saw encouraging trends in several outcomes, they failed to reach statistical significance because of the limited power. For instance, the observed power for total length of stay difference was only 17%. It is conceivable that a larger trial powered specifically for length of stayrelated outcomes may show significant results. Because subjects in this study were patients in a single midsize community teaching hospital, the results may not be generalizable to patients in, for example, tertiary‐care centers or nursing homes. However, these results may apply to a large proportion of patients in the United States, as most are treated in community hospitals. We found that many patients required 2 wrist restraints in order to protect IV lines, and this resulted in exclusion of a large proportion of potential subjects. Therefore, safe enclosures may not be appropriate for all agitated patients. They may be an ideal method of restraining patients who are not at risk of pulling out their IV line or catheters but require restraints for other reasons. This could include patients in nursing homes or rehabilitation centers.
It is also important to discuss the issue of practitioner acceptability of a newer method of restraint in acute care hospitals. As expected, we found the nursing staff was originally reluctant to use the safe enclosure, even as part of a trial. This may have been because of fear of change and having a high level of comfort with the restraint systems already in use. The setup of safe enclosures can take 10‐15 minutes, whereas the use of 2‐point soft restraints or Posey vests can be accomplished in as little as a minute. However, we found that after initial use of the safe enclosure, resistance among nurses declined. In fact, in our hospital, nurses began using safe enclosures for confused and agitated patients not enrolled in the study in order to prevent wandering and falls at night. Another difficulty reported by the nursing staff was feeling somewhat limited in their access to patients by a safe enclosure. Nurses had to open a zipped flap to access the patient to administer medication or provide food. Health care providers must remember to close the flap to avoid potential falls.
In summary, safe enclosures seem to be a safe and more acceptable alternative to the restraints currently in use in acute care hospitals. These findings should be replicated in a larger trial.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
- ,,,,.Prevalence and patterns of physical restraint use in the acute care setting.J Nurs Adm.1998;28(11):19–24.
- ,Strumpf. Myths about Elder Restraint.J Nurs Scholarsh.1990;22(2):124–128.
- , et al.Deadly restraint: a Hartford Courant investigative report.Hartford Courant1998; October 11‐15.
- ,,,,,, et al.Jt Comm J Qual Improv2001;27:605–618.
- ,,,,.Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale.Br J Addict.1989;84:1353–1357.
- ,.Development of an agitated behavior rating scale for discrete temporal observations.Nursing Meas1993;1:115–124.
- ,,,.Reliability of the Agitated Behavior Scale.J Head Trauma Rehabil.1999;14:91–96.
- University of Newcastle. The Ashton Manual. Available at: http://www.benzo.org.uk/manual/bzcha01.htm. Accessed November 15,2006.
- Postgraduate Medicine Online. Sedation and analgesia in intensive care. Available at: http://www.postgradmed.com/issues/2002/02_02/blanchard.htm. Accessed November 15,2006.
- Anti‐psychotic Comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antipsychotics%20Comparison%20Chart.pdf. Accessed November 15,2006.
- Anti‐depressant comparison chart. Available at: http://meds.queensu.ca/∼clpsych/orientation/Antidepressant%20comparison%20Chart.pdf. Accessed November 15,2006.
- ,.Use of physical and chemical restraints in medical teaching units.Can Med Assoc J.2000;162:339–340.
Copyright © 2007 Society of Hospital Medicine