User login
Adiposis Dolorosa Pain Management
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
Adiposis dolorosa (AD), or Dercum disease, is a rare disorder that was first described in 1888 and characterized by the National Organization of Rare Disorders (NORD) as a chronic pain condition of the adipose tissue generally found in patients who are overweight or obese.1,2 AD is more common in females aged 35 to 50 years and proposed to be a disease of postmenopausal women, though no prevalence studies exist.2 The etiology remains unclear.2 Several theories have been proposed, including endocrine and nervous system dysfunction, adipose tissue dysregulation, or pressure on peripheral nerves and chronic inflammation.2-4 Genetic, autoimmune, and trauma also have been proposed as a mechanism for developing the disease. Treatment modalities focusing on narcotic analgesics have been ineffective in long-term management.3
The objective of the case presentation is to report a variety of approaches for AD and their relative successes at pain control in order to assist other medical professionals who may come across patients with this rare condition.
Case Presentation
A 53-year-old male with a history of blast exposure-related traumatic brain injury, subsequent stroke with residual left hemiparesis, and seizure disorder presented with a 10-year history of nodule formation in his lower extremities causing restriction of motion and pain. The patient had previously undergone lower extremity fasciotomies for compartment syndrome with minimal pain relief. In addition, nodules over his abdomen and chest wall had been increasing over the past 5 years. He also experienced worsening fatigue, cramping, tightness, and paresthesias of the affected areas during this time. Erythema and temperature allodynia were noted in addition to an 80-pound weight gain. From the above symptoms and nodule excision showing histologic signs of lipomatous growth, a diagnosis of AD was made.
The following constitutes the approximate timetable of his treatments for 9 years. He was first diagnosed incidentally at the beginning of this period with AD during an electrodiagnostic examination. He had noticed the lipomas when he was in his 30s, but initially they were not painful. He was referred for treatment of pain to the physical medicine and rehabilitation department.
For the next 3 years, he was treated with prolotherapy. Five percent dextrose in water was injected around many of the painful lipomas in the upper extremities. He noted after the second round of neural prolotherapy that he had reduced swelling of his upper extremities and the lipomas decreased in size. He experienced mild improvement in pain and functional usage of his arms.
He continued to receive neural prolotherapy into the nodules in the arms, legs, abdomen, and chest wall. The number of painful nodules continued to increase, and the patient was started on hydrocodone 10 mg/acetaminophen 325 mg (1 tablet every 6 hours as needed) and methadone for pain relief. He was initially started on 5 mg per day of methadone and then was increased in a stepwise, gradual fashion to 10 mg in the morning and 15 mg in the evening. He transitioned to morphine sulfate, which was increased to a maximum dose of 45 mg twice daily. This medication was slowly tapered due to adverse effects (AEs), including sedation.
After weaning off morphine sulfate, the patient was started on lidocaine infusions every 3 months. Each infusion provided at least 50% pain reduction for 6 to 8 weeks. He was approved by the US Department of Veterans Affairs (VA) to have Vaser (Bausch Health, Laval, Canada) minimally invasive ultrasound liposuction treatment, performed at an outside facility. The patient was satisfied with the pain relief that he received and noted that the number of lipomas greatly diminished. However, due to funding issues, this treatment was discontinued after several months.
The patient had moderately good pain relief with methadone 5 mg in the morning, and 15 mg in the evening. However, the patient reported significant somnolence during the daytime with the regimen. Attempts to wean the patient off methadone was met with uncontrollable daytime pain. With suboptimal oral pain regimen, difficulty obtaining Vaser treatments, and limitation in frequency of neural prolotherapy, the decision was made to initiate 12 treatments of Calmare (Fairfield, CT) cutaneous electrostimulation.
During his first treatment, he had the electrodes placed on his lower extremities. The pre- and posttreatment 10-point visual analog scale (VAS) scores were 9 and 0, respectively, after the first visit. The position of the electrodes varied, depending on the location of his pain, including upper extremities and abdominal wall. During the treatment course, the patient experienced an improvement in subjective functional status. He was able to sleep in the same bed as his wife, shake hands without severe pain, and walk .25 mile, all of which he was unable to do before the electrostimulative treatment. He also reported overall improvement in emotional well-being, resumption of his hobbies (eg, playing the guitar), and social engagement. Methadone was successfully weaned off during this trial without breakthrough pain. This improvement in pain and functional status continued for several weeks; however, he had an exacerbation of his pain following a long plane flight. Due to uncertain reliability of pain relief with the procedure, the pain management service initiated a regimen of methadone 10 mg twice daily to be initiated when a procedure does not provide the desired duration of pain relief and gradually discontinued following the next interventional procedure.
The patient continued a regimen that included lidocaine infusions, neural prolotherapy, Calmare electrostimulative therapy, as well as lymphedema massage. Additionally, he began receiving weekly acupuncture treatments. He started with traditional full body acupuncture and then transitioned to battlefield acupuncture (BFA). Each acupuncture treatment provided about 50% improvement in pain on the VAS, and improved sleep for 3 days posttreatment.
However, after 18 months of the above treatment protocol, the patient experienced a general tonic-clonic seizure at home. Due to concern for the lowered seizure threshold, lidocaine infusions and methadone were discontinued. Long-acting oral morphine was initiated. The patient continued Calmare treatments and neural prolotherapy after a seizure-free interval. This regimen provided the patient with temporary pain relief but for a shorter duration than prior interventions.
Ketamine infusions were eventually initiated about 5 years after the diagnosis of AD was made, with postprocedure pain as 0/10 on the VAS. Pain relief was sustained for 3 months, with the notable AEs of hallucinations in the immediate postinfusion period. Administration consisted of the following: 500 mg of ketamine in a 500 mL bag of 0.9% NaCl. A 60-mg slow IV push was given followed by 60 mg/h increased every 15 min by 10 mg/h for a maximum dose of 150 mg/h. In a single visit the maximum total dose of ketamine administered was 500 mg. The protocol, which usually delivered 200 mg in a visit but was increased to 500 mg because the 200-mg dose was ineffective, was based on protocols at other institutions to accommodate the level of monitoring available in the Interventional Pain Clinic. The clinic also developed an infusion protocol with at least 1 month between treatments. The patient continues to undergo scheduled ketamine infusions every 14 weeks in addition to monthly BFA. The patient reported near total pain relief for about a month following ketamine infusion, with about 3 months of sustained pain relief. Each BFA session continues to provide 3 days of relief from insomnia. Calmare treatments and the neural prolotherapy regimen continue to provide effective but temporary relief from pain.
Discussion
Currently there is no curative treatment for AD. The majority of the literature is composed of case reports without summaries of potential interventions and their efficacies. AD therapies focus on symptom relief and mainly include pharmacologic and surgical intervention. In this case report several novel treatment modalities have been shown to be partially effective.
Surgical Intervention
Liposuction and lipoma resection have been described as effective only in the short term for AD.2,4-6 Hansson and colleagues suggested liposuction avulsion for sensory nerves and a portion of the proposed abnormal nerve connections between the peripheral nervous system and sensory nerves as a potential therapy for pain improvement.5 But the clinical significance of pain relief from liposuction is unclear and is contraindicated in recurrent lipomas.5
Pharmaceutical Approach
Although relief with nonsteroidal anti-inflammatory drugs and narcotic analgesics have been unpredictable, Herbst and Asare-Bediako described significant pain relief in a subset of patients with AD with a variety of oral analgesics.7,8 However, the duration of this relief was not clearly stated, and the types or medications or combinations were not discussed. Other pharmacologic agents trialed in the treatment of AD include methotrexate, infliximab, Interferon α-2b, and calcium channel modulators (pregabalin and oxcarbazepine).2,9-11 However, the mechanism and significance of pain relief from these medications remain unclear.
Subanesthesia Therapy
Lidocaine has been used as both a topical agent and an IV infusion in the treatment of chronic pain due to AD for decades. Desai and colleagues described 60% sustained pain reduction in a patient using lidocaine 5% transdermal patches.4 IV infusion of lidocaine has been described in various dosages, though the mechanism of pain relief is ambiguous, and the duration of effect is longer than the biologic half-life.2-4,9 Kosseifi and colleagues describe a patient treated with local injections of lidocaine 1% and obtained symptomatic relief for 3 weeks.9 Animal studies suggest the action of lidocaine involves the sodium channels in peripheral nerves, while another study suggested there may be an increase in sympathetic nervous system activity after the infusion of lidocaine.2,9
Ketamine infusions not previously described in the treatment of AD have long been used to treat other chronic pain syndromes (chronic cancer pain, complex regional pain syndrome [CRPS], fibromyalgia, migraine, ischemic pain, and neuropathic pain).9,12,13 Ketamine has been shown to decrease pain intensity and reduce the amount of opioid analgesic necessary to achieve pain relief, likely through the antagonism of N-methyl-D-aspartate receptors.12 A retrospective review by Patil and Anitescu described subanesthetic ketamine infusions used as a last-line therapy in refractory pain syndromes. They found ketamine reduced VAS scores from mean 8.5 prior to infusion to 0.8 after infusion in patients with CRPS and from 7.0 prior to infusion to 1.0 in patient with non-CRPS refractory pain syndromes.13 Hypertension and sedation were the most frequent AEs of ketamine infusion, though a higher incidence of hallucination and confusion were noted in non-CRPS patients. Hocking and Cousins suggest that psychotomimetic AEs of ketamine infusion may be more likely in patients with anxiety.14 However, it is important to note that ketamine infusion studies have been heterogeneous in their protocol, and only recently have standardization guidelines been proposed.
Physical Modalities
Manual lymphatic massage has been described in multiple reports for symptom relief in patients with cancer with malignant growth causing outflow lymphatic obstruction. This technique also has been used to treat the obstructive symptoms seen with the lipomatous growths of AD. Lange and colleagues described a case as providing reduction in pain and the diameter of extremities with twice weekly massage.14 Herbst and colleagues noted that patients had an equivocal response to massage, with some patients finding that it worsened the progression of lipomatous growths.7
Electrocutaneous Stimulation
In a case study by Martinenghi and colleagues, a patient with AD improved following transcutaneous frequency rhythmic electrical modulation system (FREMS) treatment.16 The treatment involved 4 cycles of 30 minutes each for 6 months. The patient had an improvement of pain on the VAS from 6.4 to 1.7 and an increase from 12 to 18 on the 100-point Barthel index scale for performance in activities of daily living, suggesting an improvement of functional independence as well.16
The MC5-A Calmare is another cutaneous electrostimulation modality that previously has been used for chronic cancer pain management. This FDA-cleared device is indicated for the treatment of various chronic pain syndromes. The device is proposed to stimulate 5 separate pain areas via cutaneous electrodes applied beyond and above the painful areas in order to “scramble” pain information and reduce perception of chronic pain intensity. Ricci and colleagues included cancer and noncancer subjects in their study and observed reduction in pain intensity by 74% (on numeric rating scale) in the entire subject group after 10 days of treatments. Further, no AEs were reported in either group, and most of the subjects were willing to continue treatment.17 However, this modality was limited by concerns with insurance coverage, access to a Calmare machine, operator training, and reproducibility of electrode placement to achieve “zero pain” as is the determinant of device treatment cycle output by the manufacturer.
Perineural Injection/Prolotherapy
Perineural injection therapy (PIT) involves the injection of dextrose solution into tissues surrounding an inflamed nerve to reduce neuropathic inflammation. The proposed source of this inflammation is the stimulation of the superficial branches of peptidergic peripheral nerves. Injections are SC and target the affected superficial nerve pathway. Pain relief is usually immediate but requires several treatments to ensure a lasting benefit. There have been no research studies or case reports on the use of PIT or prolotherapy and AD. Although there is a paucity of published literature on the efficacy of PIT, it remains an alternative modality for treatment of chronic pain syndromes. In a systematic review of prolotherapy for chronic musculoskeletal pain, Hauser and colleagues supported the use of dextrose prolotherapy to treat chronic tendinopathies, osteoarthritis of finger and knee joints, spinal and pelvic pain if conservative measures had failed. However, the efficacy on acute musculoskeletal pain was uncertain.18 In addition to the paucity of published literature, prolotherapy is not available to many patients due to lack of insurance coverage or lack of providers able to perform the procedure.
Hypobaric Pressure Therapy
Hypobaric pressure therapy has been offered as an alternative “touch-free” method for treatment of pain associated with edema. Herbst and Rutledge describe a pilot study focusing on hypobaric pressure therapy in patients with AD using a cyclic altitude conditioning system, which significantly decreased the Pain Catastrophizing Scale (tendency to catastrophize pain symptoms) in patients with AD after 5 days of therapy. VAS scores also demonstrated a linear decrease over 5 days.8
Acupuncture
There have been no research studies or case reports regarding the use of either traditional full body acupuncture or BFA in management of AD. However, prior studies have been performed that suggest that acupuncture can be beneficial in chronic pain relief. For examples, a Cochrane review by Manheimer and colleagues showed that acupuncture had a significant benefit in pain relief in subjects with peripheral joint arthritis.19 In another Cochrane review there was low-to-moderate level evidence compared with no treatment in pain relief, but moderate-level evidence that the effect of acupuncture does not differ from sham (placebo) acupuncture.20,21
Conclusion
Current therapeutic approaches to AD focus on invasive surgical intervention, chronic opiate and oral medication management. However, we have detailed several additional approaches to AD treatment. Ketamine infusions, which have long been a treatment in other chronic pain syndromes may present a viable alternative to lidocaine infusions in patients with AD. Electrocutaneous stimulation is a validated treatment of chronic pain syndromes, including chronic neuropathic pain and offers an alternative to surgical or pharmacologic management. Further, PIT offers another approach to neuropathic pain management, which has yet to be fully explored. As no standard treatment approach exists for patients with AD, multimodal therapies should be considered to optimize pain management and reduce dependency on opiate mediations.
Acknowledgments
Hunter Holmes McGuire Research Institute and the Physical Medicine and Rehabilitation Department provided the resources and facilities to make this work possible.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
1. Dercum FX. A subcutaneous dystrophy. In: University of Pennsylvania. University of Pennsylvania Medical Bulletin. Vol 1. Philadelphia, PA; University of Pennsylvania Press; 1888:140-150. Accessed October 4, 2019.
2. Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnositc criteria, diagnositic methods, classification and management. Orphanet J Rare Dis. 2012;7:1-15.
3. Amine B, Leguilchard F, Benhamou CL. Dercum’s disease (adiposis dolorosa): a new case-report. Joint Bone Spine. 2004;71(2):147-149.
4. Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum’s disease with lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9(8):1224-1226.
5. Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum’s disease (adiposis dolorosa). Pain Med. 2011;12:942-952.
6. Kosseifi S, Anaya E, Dronovalli G, Leicht S. Dercum’s disease: an unusual presentation. Pain Med. 2010;11(9):1430-1434.
7. Herbst KL, Asare-Bediako S. Adiposis dolorasa is more than painful fat. Endocrinologist. 2007;17(6):326-334.
8. Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153.
9. Lange U, Oelzner P, Uhlemann C. Dercum’s disease (lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29(1):17-22
10. Schaffer PR, Hale CS, Meehan SA, Shupack JL, Ramachandran S. Adoposis dolorosa. Dermatol Online J. 2014;20(12):1-3.
11. Singal A, Janiga JJ, Bossenbroek NM, Lim HW. Dercum’s disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venerol. 2007;21(5):717.
12. Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639-646.
13. Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012;13(2):263-269.
14. Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97(6):1730-1739.
15. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
16. Martinenghi S, Caretto A, Losio C, Scavini M, Bosi E. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94(24):e950.
17. Ricci M, Pirotti S, Scarpi E, et al. Managing chronic pain: results from an open-label study using MC5-A Calmare device. Support Care Cancer. 2012;20(2):405-412.
18. Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-159.
19. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
20. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
21. Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369.
Microprocessor Knee and Power Foot Combination in a Transfemoral Amputee
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
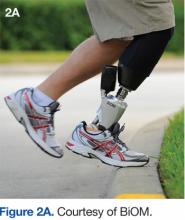
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.