User login
Pain in right shoulder • recent influenza vaccination • history of hypertension and myocardial infarction • Dx?
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
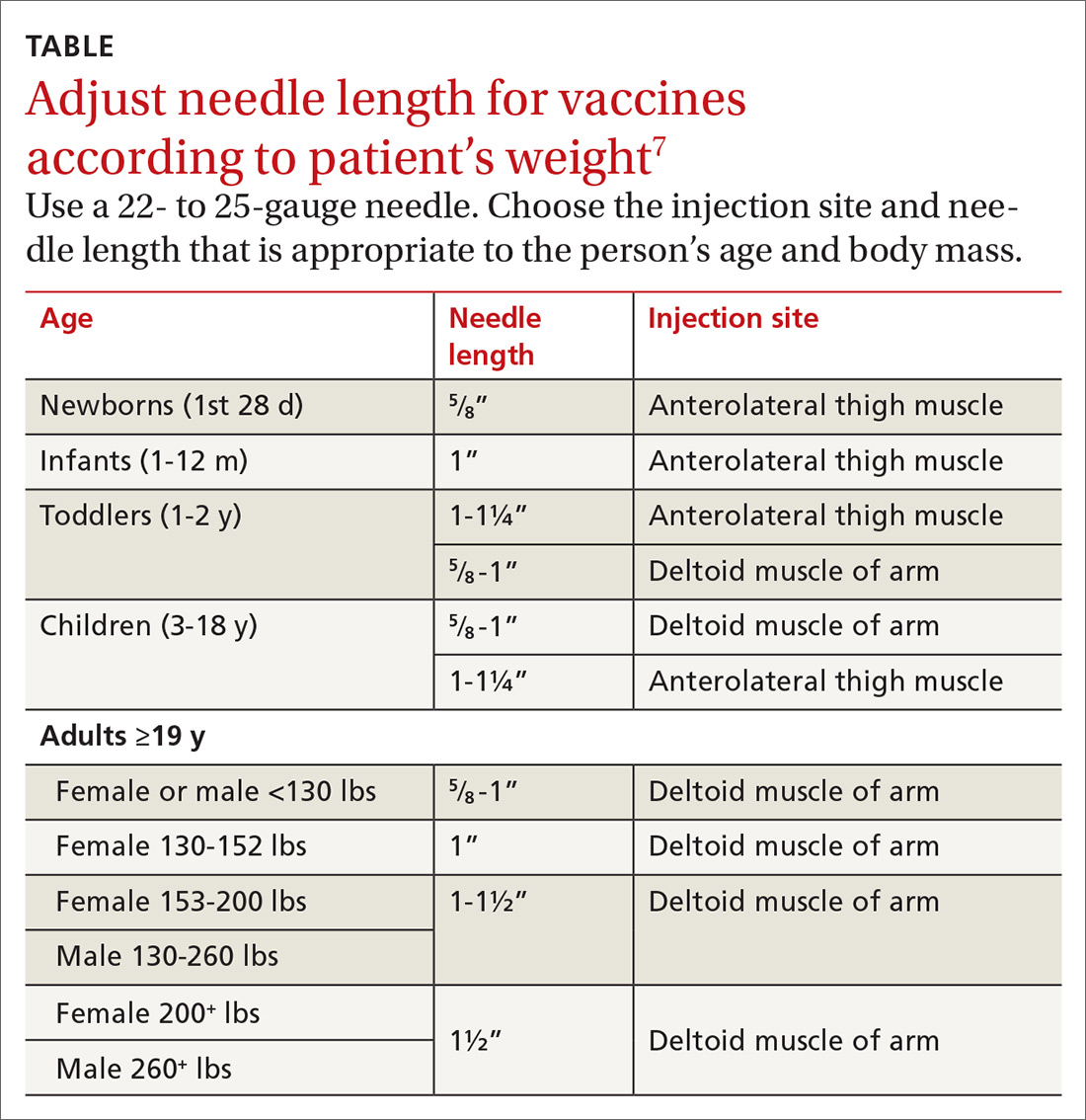
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; dustymarienarducci@gmail.com
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.