User login
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
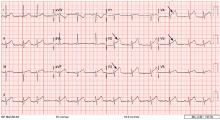
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
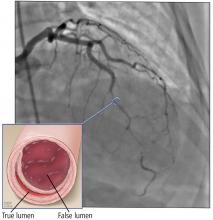
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
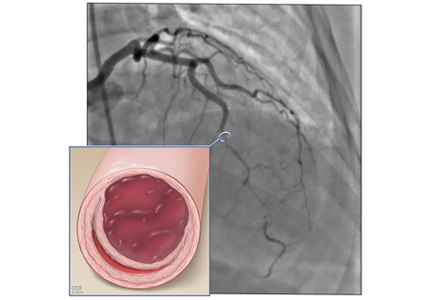
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
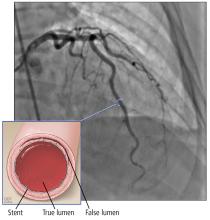
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.