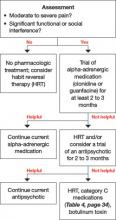
User login
Treatment-resistant OCD: There’s more we can do
Treatment-resistant OCD can be a debilitating condition. Diagnostic clarity is crucial to fully elicit symptoms and identify comorbid conditions in order to develop practical, evidence-based treatment strategies and improve the patient’s and family’s quality of life. In this article, we delineate first-line strategies for treatment-resistant OCD and then review augmentation strategies, with an emphasis on glutamate-modulating agents.
Making the diagnosis
The diagnosis of OCD is made when a patient meets DSM-5 criteria for the presence of obsessions and/or compulsions, which are defined as unwanted, distressing, intrusive, recurrent thoughts or images (obsessions) and repetitive behaviors or mental acts (compulsions).1 OCD is considered a chronic waxing and waning disorder; stress and lack of sleep lead to worsening symptoms. The hidden nature of symptoms and the reinforcement provided by the reduction in anxiety after performing a compulsion contribute to sustained illness. Eliciting symptoms from patients may be challenging due to the shame they may feel. When reviewing symptoms on the Y-BOCS, it is helpful to preface questions with statements such as “Many people report excessive concern or disgust with…” to help the patient feel understood and less anxious, rather than using direct queries, such as “Are you bothered by…?”
Consider comorbid conditions

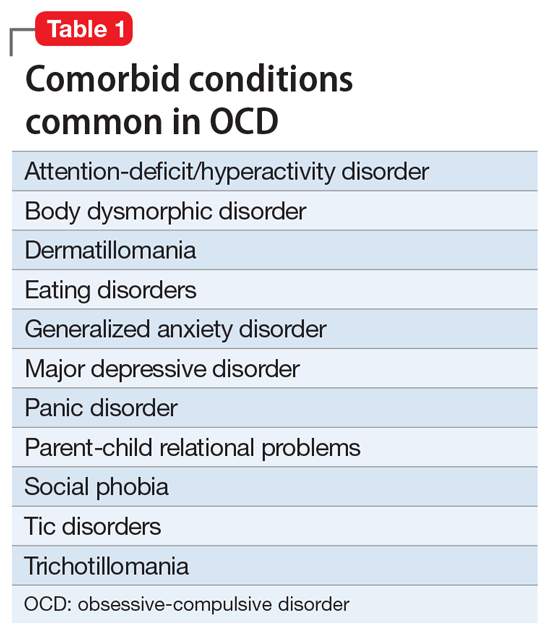
After making the initial diagnosis of OCD, it is important to assess whether the symptoms are better accounted for by another condition, and whether comorbid conditions are present (Table 1).
CASE CONTINUED
Ruling out other diagnoses
_
Initial treatment: CBT
Cognitive-behavioral therapy with exposures and response prevention (from here on referred to as CBT) has been established as a first-line, evidence-based treatment for OCD in both children and adults.2,3 For patients with treatment-resistant OCD, intensive daily CBT in a partial hospitalization or inpatient setting that is a tailor-made, patient-specific program is one of the most effective treatments, with response rates of up to 70%4-8 CBT’s advantages over medication include lower relapse rates and no known adverse effects. Unfortunately, CBT is underused9-11 due in part to a shortage of trained clinicians, and because patients may favor the ease of taking medication over the time, effort, and cost involved in CBT.
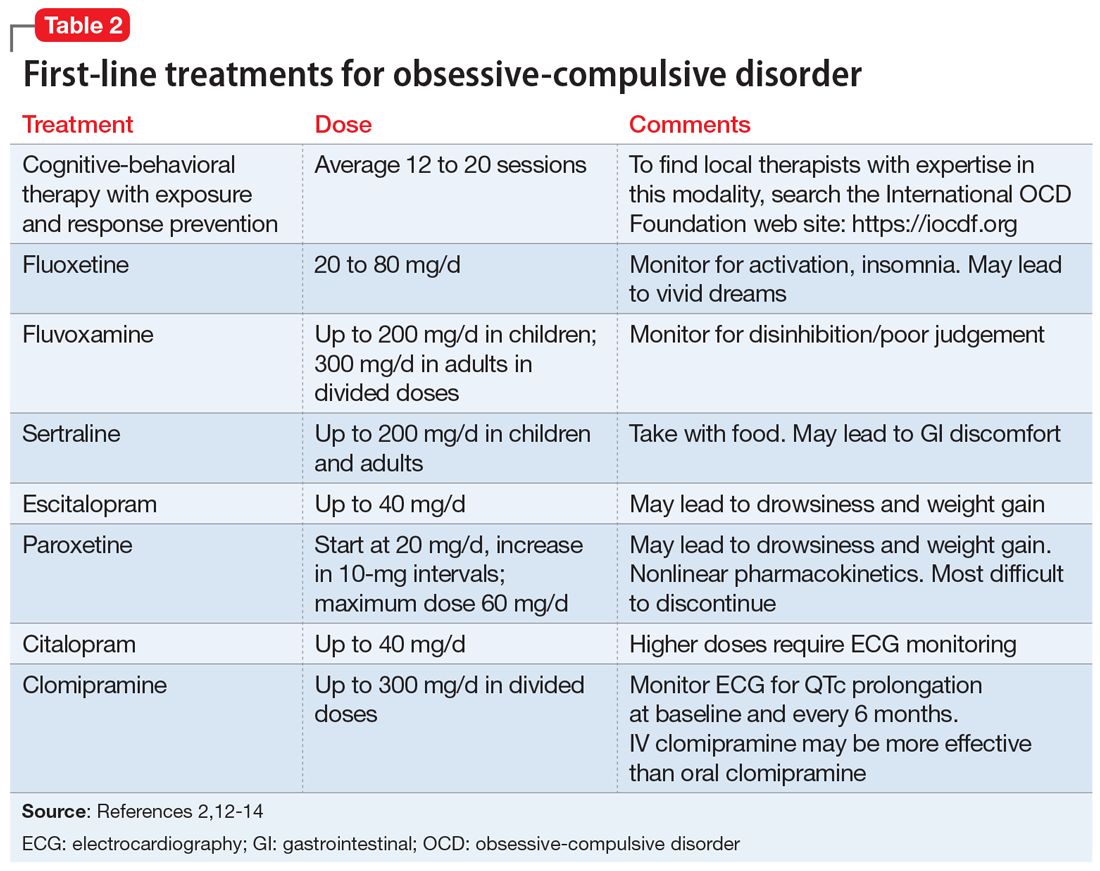
First-line pharmacologic options for treating OCD are SSRIs and clomipramine, as supported by multiple randomized controlled trials (RCTs), meta-analyses, expert guidelines, and consensus statements (Table 22,12-14). No significant difference has been found among SSRIs for the treatment of OCD in a review of 17 studies that included more than 3,000 patients.15 Treatment with SSRIs or clomipramine is effective for 50% to 60% of patients.16 Many clinicians view the combination of an SSRI and CBT as the treatment of choice for OCD.2
Continue to: Reluctance to engage in CBT
CASE CONTINUED
Reluctance to engage in CBT
To determine the next course of action, you review Mr. S’s treatment history. He has received adequate doses of 2 SSRIs and currently is taking clomipramine, 100 mg twice daily. He recently began CBT, which includes homework to help face his fears; however, Mr. S is reluctant to complete the exposure assignments, and after pausing for a few seconds as he tries to resist sending an apology email to his coworkers, he then returns to his compulsive behavior.
Facing treatment resistance
Although currently there isn’t a cure to resolve all traces of OCD, the goal of treatment is to decrease distress, interference, and the frequency of symptoms to a minimal level such that only the patients themselves are aware of symptoms. In broad terms, “response” has been defined as a decrease in symptoms, and “remission” has been defined as minimal symptoms after treatment.
Close to half of adults treated for OCD respond well to standard-of-care treatment (CBT and/or an SSRI), while the other 50% are considered partial responders or nonresponders.2 For patients with OCD, researchers often define “treatment response” as a ≥25% reduction in symptom severity score on the Y-BOCS. Approximately 30% of adults with OCD do not respond substantially to the first-line treatments, and even those who are defined as “responders” in research studies typically continue to have significant symptoms that impact their quality of life.2 In children, a clinical definition for treatment-refractory OCD has been presented as failing to achieve adequate symptom relief despite receiving an adequate course of CBT and at least 2 adequate trials of an SSRI or clomipramine.17 In the Pediatric OCD Treatment Study (POTS) trial, >46% of youth did not achieve remission from their OCD symptoms, even after receiving evidence-based care provided by experienced clinicians (combined treatment with CBT and an SSRI).18
_
Challenges in psychotherapy
Compassion is a key element in developing rapport with patients to help them face increasingly more challenging exposures. Making OCD the problem, not the person, is an essential element in helping patients move forward. Some clinicians may become frustrated with patients when treatment is not moving along well, referring to resistance, denial, or sabotage. According to March and Mulle,19 these terms lack the recognition and compassion that exposures are inherently difficult.19
Another challenge for therapists is if the patient’s presenting symptoms are personally offensive or a sensitive topic. For example, a therapist who is disgusted by public restrooms will find it difficult to tolerate the risks associated with exposure to germs and support a patient in touching objects in the restroom. Therapists also may be challenged when the patient’s fears align with the therapist’s religious beliefs. In these situations, consider transferring care to another therapist.
Family members need to learn about the nature of the illness and their roles in helping patients improve. Family members may unknowingly enable symptoms or criticize patients for their lack of motivation, which can lead to conflict in the home. Family dysfunction can in turn worsen OCD symptoms.
The most likely cause of lack of response to therapy is inexpert CBT.19 Deep breathing and relaxation training have been used as an active placebo in studies20; in a meta-analysis examining the effective components of CBT, studies that added relaxation training were not more effective than those that employed exposures alone.21 Patients receiving CBT should be able to articulate the hierarchical approach used to gradually face their fears.
Continue to: Pharmacologic augmentation strategies
Pharmacologic augmentation strategies
Selective serotonin reuptake inhibitors. While most OCD research trials have assessed SSRIs in 12-week studies, clinicians may consider extending SSRI treatment for an additional 12 weeks for nonresponders because some patients will continue to make gains. In the past, it was generally believed that higher doses of SSRIs are needed for treating OCD than for treating major depressive disorder. For instance, greater improvement was seen with 250 to 400 mg/d of sertraline compared with 200 mg/d22 and with escitalopram after an increase of dose up to 50 mg/d.23 However, more recently, this notion of higher doses being necessary for treatment response has been called into question. For example, a study of escitalopram found similar responses to 10 mg/d vs 20 mg/d after 24 weeks.24 A meta-analysis of adult studies of SSRIs for OCD supported higher doses as being more effective, but noted that the drop-out rate from treatment was greater in patients treated with higher doses.25 As a note of caution, long-term, high-dose maintenance therapy increases the risk of adverse reactions.26
Following a failed treatment with a first SSRI, it remains debatable as to what ought to be the second pharmacologic treatment. Although clomipramine is often reserved for treatment after 2 failed trials of an SSRI due to its greater risk of adverse effects, in an open-label study, switching from an SSRI to clomipramine led to greater response than switching from one SSRI to another.27 On the other hand, while meta-analyses have reported greater treatment effect for oral clomipramine than for SSRIs, direct head-to-head comparisons have not supported this notion.28 To get the best of both worlds, some clinicians employ a strategy of combining clomipramine with an SSRI, while monitoring for adverse effects and interactions such as serotonin syndrome.29-31
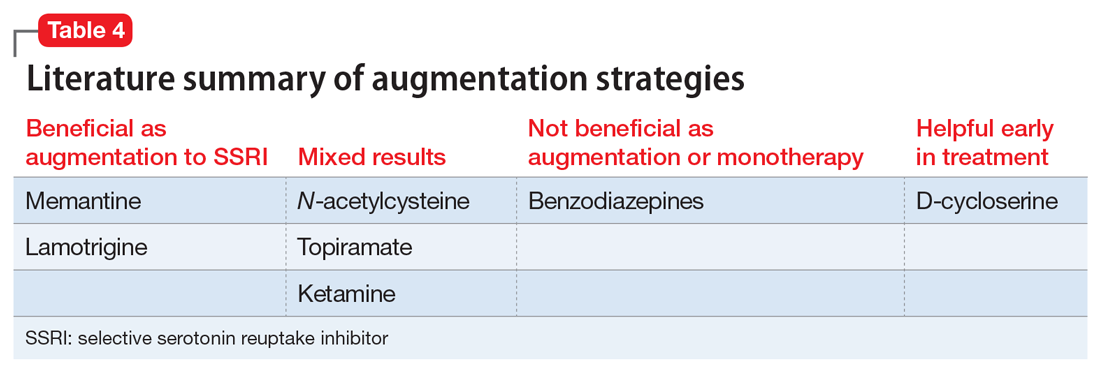
Benzodiazepines. Although benzodiazepines are useful for brief treatment of an anxiety disorder (eg, for a person with a fear of heights who needs to take an airplane),32 they have not been shown to be effective for OCD33 or as augmentation to an SSRI.34
N-acetylcysteine (NAC). Two RCTs of adults with OCD who received adjunctive NAC, 3 g/d in divided doses, found no significant difference in the treatment arms by the conclusion of 16 weeks—either both groups improved, or both groups failed to improve.35,36 In a 10-week study of patients with moderate to severe OCD symptoms, NAC, 2 g/d, as augmentation to fluvoxamine, 200 mg/d, showed a significant time x interaction in the treatment group.37 No follow-up information is available, however.
In a multicenter RCT of NAC given to children and adolescents with OCD as augmentation to citalopram, symptoms decreased and the quality-of-life score improved, with a large treatment effect size in the NAC group.38 However, in a study aimed at examining NAC in youth with Tourette syndrome, OCD symptoms were measured as a secondary outcome and there was no benefit of NAC over placebo.39
Memantine. Four 8- to 12-week RCTs in adults with OCD favored adjunctive memantine, 20 mg/d, taken with an SSRI, over placebo.40-43 A small study suggests that patients with OCD may be more likely to respond to memantine than patients with generalized anxiety disorder.44 Case reports have noted that memantine has been beneficial for pediatric patients with refractory OCD.45
Continue to: Topiramate
Topiramate. Three 12-week RCTs examined topiramate augmentation at 100 to 400 mg/d in patients with OCD who had failed at least 1 previous trial of an SSRI. The earliest study was most encouraging: Y-BOCS scores decreased by 32% in the topiramate group but by only 2.4% in the placebo group.46 However, the other 2 studies found no difference in the final OCD symptom severity score between active treatment and placebo groups,47,48 and the use of topiramate, particularly at higher doses, was limited by its adverse effects.
Lamotrigine. Initially, lamotrigine augmentation of SSRIs in OCD did not appear to be helpful.49 More recently, several case studies reported that lamotrigine, 100 to 200 mg/d, added to paroxetine or clomipramine, resulted in dramatic improvement in Y-BOCS scores for patients with long-standing refractory symptoms.50,51 In a retrospective review of 22 patients who received augmentation with lamotrigine, 150 mg/d, 20 had a significant response; the mean decrease in Y-BOCS score was 67%.52 Finally, in a 16-week RCT, lamotrigine, 100 mg/d, added to an SSRI led to a significant decrease in both Y-BOCS score and depressive symptoms while also improving semantic fluency.53
Ketamine. Ketamine is drawing increased attention for its nearly instantaneous antidepressant effect that lasts for up to 2 weeks after a single infusion.54 In a study of 15 medication-free adults with continuous intrusive obsessions, 4 of 8 patients who received a single IV infusion of ketamine, 0.5 mg/kg, met the criteria for treatment response (>35% reduction in Y-BOCS score measured 1 week later); none of the patients who received a placebo infusion of saline met this criteria.55 A small open-label trial of 10 treatment-refractory patients found that an infusion of ketamine, 0.5 mg/kg, was beneficial for comorbid depression but had only a minimal effect on OCD symptoms measured 3 days post-infusion.56 A short-term follow-up on these patients revealed dysphoria in some responders.57
D-cycloserine. The idea of using a pharmacologic agent to increase the speed or efficacy of behavioral therapy is intriguing. Proof of concept was demonstrated in a study that found that giving D-cycloserine prior to computerized exposure therapy significantly improved clinical response in patients with acrophobia.58 However, using this approach to treating OCD netted mixed results; D-cycloserine was found to be most helpful during early stages of treatment.59,60
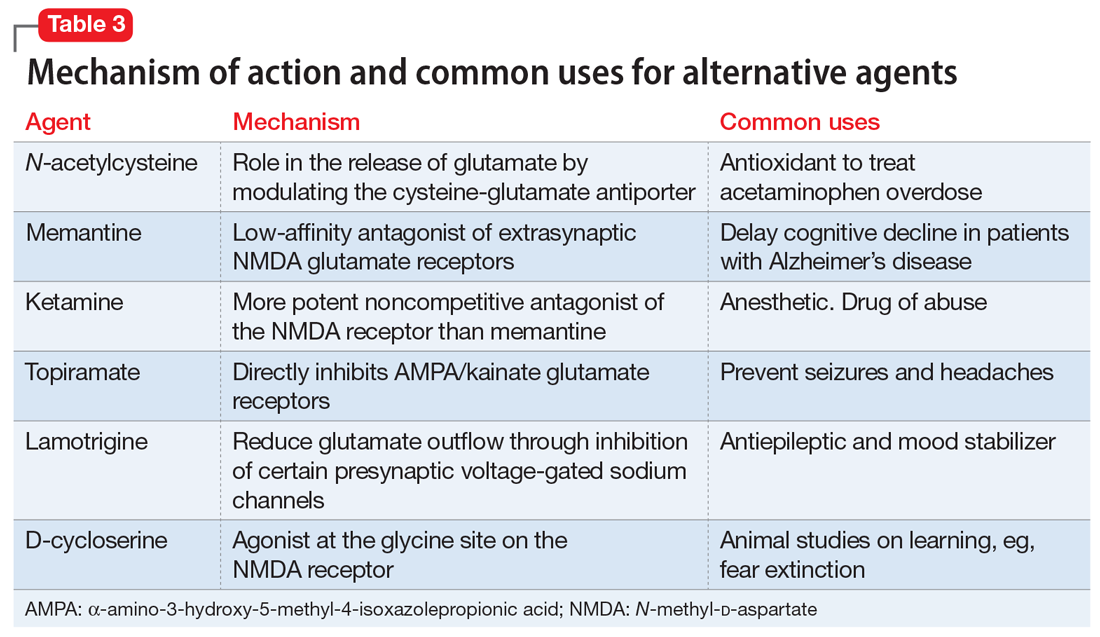
Table 3 outlines the mechanisms of action and common uses for NAC, memantine, ketamine, topiramate, lamotrigine, and D-cycloserine. Table 4 summarizes the literature on the efficacy of some of the augmentation strategies for treating OCD described in this article.
Continue to: Alternative strategies
Alternative strategies
Augmentation strategies with neuroleptics,61 transcranial magnetic stimulation,62 and deep brain stimulation63 have recently been reviewed. Space limitations preclude a comprehensive review of these strategies, but in a cross-sectional study of augmentation strategies in OCD, no difference was found in terms of symptom severity between those prescribed SSRI monotherapy or augmentation with neuroleptics, benzodiazepines, or antidepressants.64
CASE CONTINUED
Progress in CBT
Mr. S agrees to a trial of NAC as an augmentation strategy, but after 8 weeks of treatment with NAC, 600 mg twice daily, his Y-BOCS had declined by only 2 points. He also complains of nausea and does not want to increase the dose. You discontinue NAC and opt to further explore his reaction to CBT. Mr. S shares that he has been seeing his psychologist only once every 3 weeks because he does not want to miss work. You encourage him to increase to weekly CBT sessions, and you obtain his permission to contact his therapist and his family members. Fortunately, his therapist is highly qualified, but unfortunately, Mr. S’s father has been sending him multiple critical emails about not advancing at his job and for being “lazy” at work. You schedule a session with Mr. S and his father. Great progress is made after Mr. S and his father both share their frustrations and come to understand and appreciate each other’s struggles. Four weeks later, after weekly CBT appointments, Mr. S has a Y-BOCS of 18 and spends <2 hours/d checking emails for errors and apologizing.
Bottom Line
It is unrealistic to expect OCD symptoms to be cured. Many ‘treatment-resistant’ patients have not received properly delivered cognitive-behavioral therapy, and this first-line treatment modality should be considered in every eligible patient, and augmented with a selective serotonin reuptake inhibitor (SSRI) when needed. Glutamatergic agents, in turn, can augment SSRIs.
Related Resources
- Yale-Brown Obsessive-Compulsive Scale. https://iocdf.org/ wp-content/uploads/2014/08/Assessment-Tools.pdf.
- The International OCD Foundation. https://iocdf.org.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Ketamine • Ketalar
Lamotrigine • Lamictal
Memantine • Namenda
Paroxetine • Paxil
Sertraline • Zoloft
Topiramate • Topomax
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry; 2007;164(suppl 7):5-53.
3. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
4. Bystritsky A, Munford PR, Rosen RM, et al. A preliminary study of partial hospital management of severe obsessive-compulsive disorder. Psychiatr Serv. 1996;47(2):170-174.
5. Calvocoressi L, McDougle CI, Wasylink S, et al. Inpatient treatment of patients with severe obsessive-compulsive disorder. Hosp Community Psychiatry. 1993;44(12):1150-1154.
6. Eddy KT, Dutra L, Bradley R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
7. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
8. Simpson HB, Huppert JD, Petkova E, et al. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(2):269-276.
9. Marques L, LeBlanc NJ, Weingarden HM, et al. Barriers to treatment and service utilization in an internet sample of individuals with obsessive-compulsive symptoms. Depress Anxiety. 2010;27(5):470-475.
10. Goodwin R, Koenen KC, Hellman F, et al. Helpseeking and access to mental health treatment for obsessive-compulsive disorder. Acta Psychiatr Scand. 2002;106(2):143-149.
11. Kohn R, Saxena S, Levav I, et al. The treatment gap in mental health care. Bull World Health Organ. 2004;82(11):858-866.
12. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439.
13. Lovell K, Bee P. Implementing the NICE OCD/BDD guidelines. Psychol Psychother. 2008;81(Pt 4):365-376.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84.
15. Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;(1):CD001765.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
17. Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in children. J Am Acad Child Adolesc Psychiatry. 2015;54(4):251-262.
18. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
19. March JS, Mulle K. OCD in children and adolescents: a cognitive-behavioral treatment manual. New York, NY: Guilford Press; 1998.
20. Marks IM. Fears, phobias, and rituals: Panic, anxiety, and their disorders. 1987, New York, NY: Oxford University Press; 1987.
21. Ale CM, McCarthy DM, Rothschild LM, et al. Components of cognitive behavioral therapy related to outcome in childhood anxiety disorders. Clin Child Fam Psychol Rev. 2015;18(3):240-251.
22. Ninan PT, Koran LM, Kiev A, et al. High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. J Clin Psychiatry. 2006;67(1):15-22.
23. Rabinowitz I, Baruch Y, Barak Y. High-dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2008;23(1):49-53.
24. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
25. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
26. Sayyah M, Majzoob S, Sayyah M. Metabolic and toxicological considerations for obsessive-compulsive disorder drug therapy. Expert Opin Drug Metab Toxicol. 2013;9(6):657-673.
27. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry. 2002;63(suppl 6):20-29.
28. Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005;8(1):107-129.
29. Marazziti D, Golia F, Consoli G, et al. Effectiveness of long-term augmentation with citalopram to clomipramine in treatment-resistant OCD patients. CNS Spectr. 2008;13(11):971-976.
30. Browne M, Horn E, Jones TT. The benefits of clomipramine-fluoxetine combination in obsessive compulsive disorder. Can J Psychiatry. 1993;38(4):242-243.
31. Ravizza L, Barzega G, Bellino S, et al. Drug treatment of obsessive-compulsive disorder (OCD): long-term trial with clomipramine and selective serotonin reuptake inhibitors (SSRIs). Psychopharmacol Bull. 1996;32(1):167-173.
32. Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci. 2011;13(4):423-437.
33. Hollander E, Kaplan A, Stahl SM. A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J Biol Psychiatry. 2003;4(1):30-34.
34. Crockett BA, Churchill E, Davidson JR. A double-blind combination study of clonazepam with sertraline in obsessive-compulsive disorder. Ann Clin Psychiatry. 2004;16(3):127-132.
35. Costa DLC, Diniz JB, Requena G, et al. Randomized, double-blind, placebo-controlled trial of n-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7):e766-e773.
36. Sarris J, Oliver G, Camfield DA, et al. N-Acetyl Cysteine (NAC) in the treatment of obsessive-compulsive disorder: a 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29(9):801-809.
37. Paydary K, Akamaloo A, Ahmadipour A, et al. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016;41(2):214-219.
38. Ghanizadeh A, Mohammadi MR, Bahraini S, et al. Efficacy of N-acetylcysteine augmentation on obsessive compulsive disorder: a multicenter randomized double blind placebo controlled clinical trial. Iran J Psychiatry. 2017;12(2):134-141.
39. Bloch MH, Panza KE, Yaffa A, et al. N-acetylcysteine in the treatment of pediatric tourette syndrome: randomized, double-blind, placebo-controlled add-on trial. J Child Adolesc Psychopharmacol. 2016;26(4):327-334.
40. Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47(2):175-180.
41. Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34-39.
42. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2017. doi: 10.1055/s-0043-120268. [Epub ahead of print].
43. Haghighi M, Jahangard L, Mohammad-Beigi H, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013;228(4):633-640.
44. Feusner JD, Kerwin L, Saxena S, et al. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81-93.
45. Hezel DM, Beattie K, Stewart SE. Memantine as an augmenting agent for severe pediatric OCD. Am J Psychiatry. 2009;166(2):237.
46. Mowla A, Khajeian AM, Sahraian A, et al. topiramate augmentation in resistant ocd: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15(11):613-617.
47. Berlin H, Koran LM, Jenike MA, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(5):716-721.
48. Afshar H, Akuchekian S, Mahaky B, et al. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(10):976-981.
49. Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry. 2000;34(3):527-528.
50. Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry. 2013;2013:612459.
51. Uzun O. Lamotrigine as an augmentation agent in treatment-resistant obsessive-compulsive disorder: a case report. J Psychopharmacol. 2010;24(3):425-427.
52. Hussain A, Dar MA, Wani RA, et al. Role of lamotrigine augmentation in treatment-resistant obsessive compulsive disorder: a retrospective case review from South Asia. Indian J Psychol Med. 2015;37(2):154-158.
53. Bruno A, Micò U, Pandolfo G, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol. 2012;26(11):1456-1462.
54. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73(12):113311-41.
55. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483.
56. Bloch MH, Wasylink S, Landeros-Weisenberger A,, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964-970.
57. Niciu MJ, Grunschel BD, Corlett PR, et al. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
58. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136-1144.
59. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
60. Xia J, Du Y, Han J, et al. D-cycloserine augmentation in behavioral therapy for obsessive-compulsive disorder: a meta-analysis. Drug Des Devel Ther. 2015;9:2101-2117.
61. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:317.
62. Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. 2017;33(6):747-756.
63. Naesström, M, Blomstedt P, Bodlund O. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Nord J Psychiatry. 2016;70(7):483-491.
64. Van Ameringen M, Simpson W, Patterson B, et al. Pharmacological treatment strategies in obsessive compulsive disorder: A cross-sectional view in nine international OCD centers. J Psychopharmacol, 2014;28(6):596-602.
Treatment-resistant OCD can be a debilitating condition. Diagnostic clarity is crucial to fully elicit symptoms and identify comorbid conditions in order to develop practical, evidence-based treatment strategies and improve the patient’s and family’s quality of life. In this article, we delineate first-line strategies for treatment-resistant OCD and then review augmentation strategies, with an emphasis on glutamate-modulating agents.
Making the diagnosis
The diagnosis of OCD is made when a patient meets DSM-5 criteria for the presence of obsessions and/or compulsions, which are defined as unwanted, distressing, intrusive, recurrent thoughts or images (obsessions) and repetitive behaviors or mental acts (compulsions).1 OCD is considered a chronic waxing and waning disorder; stress and lack of sleep lead to worsening symptoms. The hidden nature of symptoms and the reinforcement provided by the reduction in anxiety after performing a compulsion contribute to sustained illness. Eliciting symptoms from patients may be challenging due to the shame they may feel. When reviewing symptoms on the Y-BOCS, it is helpful to preface questions with statements such as “Many people report excessive concern or disgust with…” to help the patient feel understood and less anxious, rather than using direct queries, such as “Are you bothered by…?”

Consider comorbid conditions

After making the initial diagnosis of OCD, it is important to assess whether the symptoms are better accounted for by another condition, and whether comorbid conditions are present (Table 1).
CASE CONTINUED
Ruling out other diagnoses
_
Initial treatment: CBT
Cognitive-behavioral therapy with exposures and response prevention (from here on referred to as CBT) has been established as a first-line, evidence-based treatment for OCD in both children and adults.2,3 For patients with treatment-resistant OCD, intensive daily CBT in a partial hospitalization or inpatient setting that is a tailor-made, patient-specific program is one of the most effective treatments, with response rates of up to 70%4-8 CBT’s advantages over medication include lower relapse rates and no known adverse effects. Unfortunately, CBT is underused9-11 due in part to a shortage of trained clinicians, and because patients may favor the ease of taking medication over the time, effort, and cost involved in CBT.
First-line pharmacologic options for treating OCD are SSRIs and clomipramine, as supported by multiple randomized controlled trials (RCTs), meta-analyses, expert guidelines, and consensus statements (Table 22,12-14). No significant difference has been found among SSRIs for the treatment of OCD in a review of 17 studies that included more than 3,000 patients.15 Treatment with SSRIs or clomipramine is effective for 50% to 60% of patients.16 Many clinicians view the combination of an SSRI and CBT as the treatment of choice for OCD.2

Continue to: Reluctance to engage in CBT
CASE CONTINUED
Reluctance to engage in CBT
To determine the next course of action, you review Mr. S’s treatment history. He has received adequate doses of 2 SSRIs and currently is taking clomipramine, 100 mg twice daily. He recently began CBT, which includes homework to help face his fears; however, Mr. S is reluctant to complete the exposure assignments, and after pausing for a few seconds as he tries to resist sending an apology email to his coworkers, he then returns to his compulsive behavior.
Facing treatment resistance
Although currently there isn’t a cure to resolve all traces of OCD, the goal of treatment is to decrease distress, interference, and the frequency of symptoms to a minimal level such that only the patients themselves are aware of symptoms. In broad terms, “response” has been defined as a decrease in symptoms, and “remission” has been defined as minimal symptoms after treatment.
Close to half of adults treated for OCD respond well to standard-of-care treatment (CBT and/or an SSRI), while the other 50% are considered partial responders or nonresponders.2 For patients with OCD, researchers often define “treatment response” as a ≥25% reduction in symptom severity score on the Y-BOCS. Approximately 30% of adults with OCD do not respond substantially to the first-line treatments, and even those who are defined as “responders” in research studies typically continue to have significant symptoms that impact their quality of life.2 In children, a clinical definition for treatment-refractory OCD has been presented as failing to achieve adequate symptom relief despite receiving an adequate course of CBT and at least 2 adequate trials of an SSRI or clomipramine.17 In the Pediatric OCD Treatment Study (POTS) trial, >46% of youth did not achieve remission from their OCD symptoms, even after receiving evidence-based care provided by experienced clinicians (combined treatment with CBT and an SSRI).18
_
Challenges in psychotherapy
Compassion is a key element in developing rapport with patients to help them face increasingly more challenging exposures. Making OCD the problem, not the person, is an essential element in helping patients move forward. Some clinicians may become frustrated with patients when treatment is not moving along well, referring to resistance, denial, or sabotage. According to March and Mulle,19 these terms lack the recognition and compassion that exposures are inherently difficult.19
Another challenge for therapists is if the patient’s presenting symptoms are personally offensive or a sensitive topic. For example, a therapist who is disgusted by public restrooms will find it difficult to tolerate the risks associated with exposure to germs and support a patient in touching objects in the restroom. Therapists also may be challenged when the patient’s fears align with the therapist’s religious beliefs. In these situations, consider transferring care to another therapist.
Family members need to learn about the nature of the illness and their roles in helping patients improve. Family members may unknowingly enable symptoms or criticize patients for their lack of motivation, which can lead to conflict in the home. Family dysfunction can in turn worsen OCD symptoms.
The most likely cause of lack of response to therapy is inexpert CBT.19 Deep breathing and relaxation training have been used as an active placebo in studies20; in a meta-analysis examining the effective components of CBT, studies that added relaxation training were not more effective than those that employed exposures alone.21 Patients receiving CBT should be able to articulate the hierarchical approach used to gradually face their fears.
Continue to: Pharmacologic augmentation strategies
Pharmacologic augmentation strategies
Selective serotonin reuptake inhibitors. While most OCD research trials have assessed SSRIs in 12-week studies, clinicians may consider extending SSRI treatment for an additional 12 weeks for nonresponders because some patients will continue to make gains. In the past, it was generally believed that higher doses of SSRIs are needed for treating OCD than for treating major depressive disorder. For instance, greater improvement was seen with 250 to 400 mg/d of sertraline compared with 200 mg/d22 and with escitalopram after an increase of dose up to 50 mg/d.23 However, more recently, this notion of higher doses being necessary for treatment response has been called into question. For example, a study of escitalopram found similar responses to 10 mg/d vs 20 mg/d after 24 weeks.24 A meta-analysis of adult studies of SSRIs for OCD supported higher doses as being more effective, but noted that the drop-out rate from treatment was greater in patients treated with higher doses.25 As a note of caution, long-term, high-dose maintenance therapy increases the risk of adverse reactions.26
Following a failed treatment with a first SSRI, it remains debatable as to what ought to be the second pharmacologic treatment. Although clomipramine is often reserved for treatment after 2 failed trials of an SSRI due to its greater risk of adverse effects, in an open-label study, switching from an SSRI to clomipramine led to greater response than switching from one SSRI to another.27 On the other hand, while meta-analyses have reported greater treatment effect for oral clomipramine than for SSRIs, direct head-to-head comparisons have not supported this notion.28 To get the best of both worlds, some clinicians employ a strategy of combining clomipramine with an SSRI, while monitoring for adverse effects and interactions such as serotonin syndrome.29-31
Benzodiazepines. Although benzodiazepines are useful for brief treatment of an anxiety disorder (eg, for a person with a fear of heights who needs to take an airplane),32 they have not been shown to be effective for OCD33 or as augmentation to an SSRI.34
N-acetylcysteine (NAC). Two RCTs of adults with OCD who received adjunctive NAC, 3 g/d in divided doses, found no significant difference in the treatment arms by the conclusion of 16 weeks—either both groups improved, or both groups failed to improve.35,36 In a 10-week study of patients with moderate to severe OCD symptoms, NAC, 2 g/d, as augmentation to fluvoxamine, 200 mg/d, showed a significant time x interaction in the treatment group.37 No follow-up information is available, however.
In a multicenter RCT of NAC given to children and adolescents with OCD as augmentation to citalopram, symptoms decreased and the quality-of-life score improved, with a large treatment effect size in the NAC group.38 However, in a study aimed at examining NAC in youth with Tourette syndrome, OCD symptoms were measured as a secondary outcome and there was no benefit of NAC over placebo.39
Memantine. Four 8- to 12-week RCTs in adults with OCD favored adjunctive memantine, 20 mg/d, taken with an SSRI, over placebo.40-43 A small study suggests that patients with OCD may be more likely to respond to memantine than patients with generalized anxiety disorder.44 Case reports have noted that memantine has been beneficial for pediatric patients with refractory OCD.45
Continue to: Topiramate
Topiramate. Three 12-week RCTs examined topiramate augmentation at 100 to 400 mg/d in patients with OCD who had failed at least 1 previous trial of an SSRI. The earliest study was most encouraging: Y-BOCS scores decreased by 32% in the topiramate group but by only 2.4% in the placebo group.46 However, the other 2 studies found no difference in the final OCD symptom severity score between active treatment and placebo groups,47,48 and the use of topiramate, particularly at higher doses, was limited by its adverse effects.
Lamotrigine. Initially, lamotrigine augmentation of SSRIs in OCD did not appear to be helpful.49 More recently, several case studies reported that lamotrigine, 100 to 200 mg/d, added to paroxetine or clomipramine, resulted in dramatic improvement in Y-BOCS scores for patients with long-standing refractory symptoms.50,51 In a retrospective review of 22 patients who received augmentation with lamotrigine, 150 mg/d, 20 had a significant response; the mean decrease in Y-BOCS score was 67%.52 Finally, in a 16-week RCT, lamotrigine, 100 mg/d, added to an SSRI led to a significant decrease in both Y-BOCS score and depressive symptoms while also improving semantic fluency.53
Ketamine. Ketamine is drawing increased attention for its nearly instantaneous antidepressant effect that lasts for up to 2 weeks after a single infusion.54 In a study of 15 medication-free adults with continuous intrusive obsessions, 4 of 8 patients who received a single IV infusion of ketamine, 0.5 mg/kg, met the criteria for treatment response (>35% reduction in Y-BOCS score measured 1 week later); none of the patients who received a placebo infusion of saline met this criteria.55 A small open-label trial of 10 treatment-refractory patients found that an infusion of ketamine, 0.5 mg/kg, was beneficial for comorbid depression but had only a minimal effect on OCD symptoms measured 3 days post-infusion.56 A short-term follow-up on these patients revealed dysphoria in some responders.57
D-cycloserine. The idea of using a pharmacologic agent to increase the speed or efficacy of behavioral therapy is intriguing. Proof of concept was demonstrated in a study that found that giving D-cycloserine prior to computerized exposure therapy significantly improved clinical response in patients with acrophobia.58 However, using this approach to treating OCD netted mixed results; D-cycloserine was found to be most helpful during early stages of treatment.59,60
Table 3 outlines the mechanisms of action and common uses for NAC, memantine, ketamine, topiramate, lamotrigine, and D-cycloserine. Table 4 summarizes the literature on the efficacy of some of the augmentation strategies for treating OCD described in this article.

Continue to: Alternative strategies
Alternative strategies
Augmentation strategies with neuroleptics,61 transcranial magnetic stimulation,62 and deep brain stimulation63 have recently been reviewed. Space limitations preclude a comprehensive review of these strategies, but in a cross-sectional study of augmentation strategies in OCD, no difference was found in terms of symptom severity between those prescribed SSRI monotherapy or augmentation with neuroleptics, benzodiazepines, or antidepressants.64

CASE CONTINUED
Progress in CBT
Mr. S agrees to a trial of NAC as an augmentation strategy, but after 8 weeks of treatment with NAC, 600 mg twice daily, his Y-BOCS had declined by only 2 points. He also complains of nausea and does not want to increase the dose. You discontinue NAC and opt to further explore his reaction to CBT. Mr. S shares that he has been seeing his psychologist only once every 3 weeks because he does not want to miss work. You encourage him to increase to weekly CBT sessions, and you obtain his permission to contact his therapist and his family members. Fortunately, his therapist is highly qualified, but unfortunately, Mr. S’s father has been sending him multiple critical emails about not advancing at his job and for being “lazy” at work. You schedule a session with Mr. S and his father. Great progress is made after Mr. S and his father both share their frustrations and come to understand and appreciate each other’s struggles. Four weeks later, after weekly CBT appointments, Mr. S has a Y-BOCS of 18 and spends <2 hours/d checking emails for errors and apologizing.
Bottom Line
It is unrealistic to expect OCD symptoms to be cured. Many ‘treatment-resistant’ patients have not received properly delivered cognitive-behavioral therapy, and this first-line treatment modality should be considered in every eligible patient, and augmented with a selective serotonin reuptake inhibitor (SSRI) when needed. Glutamatergic agents, in turn, can augment SSRIs.
Related Resources
- Yale-Brown Obsessive-Compulsive Scale. https://iocdf.org/ wp-content/uploads/2014/08/Assessment-Tools.pdf.
- The International OCD Foundation. https://iocdf.org.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Ketamine • Ketalar
Lamotrigine • Lamictal
Memantine • Namenda
Paroxetine • Paxil
Sertraline • Zoloft
Topiramate • Topomax
Treatment-resistant OCD can be a debilitating condition. Diagnostic clarity is crucial to fully elicit symptoms and identify comorbid conditions in order to develop practical, evidence-based treatment strategies and improve the patient’s and family’s quality of life. In this article, we delineate first-line strategies for treatment-resistant OCD and then review augmentation strategies, with an emphasis on glutamate-modulating agents.
Making the diagnosis
The diagnosis of OCD is made when a patient meets DSM-5 criteria for the presence of obsessions and/or compulsions, which are defined as unwanted, distressing, intrusive, recurrent thoughts or images (obsessions) and repetitive behaviors or mental acts (compulsions).1 OCD is considered a chronic waxing and waning disorder; stress and lack of sleep lead to worsening symptoms. The hidden nature of symptoms and the reinforcement provided by the reduction in anxiety after performing a compulsion contribute to sustained illness. Eliciting symptoms from patients may be challenging due to the shame they may feel. When reviewing symptoms on the Y-BOCS, it is helpful to preface questions with statements such as “Many people report excessive concern or disgust with…” to help the patient feel understood and less anxious, rather than using direct queries, such as “Are you bothered by…?”

Consider comorbid conditions

After making the initial diagnosis of OCD, it is important to assess whether the symptoms are better accounted for by another condition, and whether comorbid conditions are present (Table 1).
CASE CONTINUED
Ruling out other diagnoses
_
Initial treatment: CBT
Cognitive-behavioral therapy with exposures and response prevention (from here on referred to as CBT) has been established as a first-line, evidence-based treatment for OCD in both children and adults.2,3 For patients with treatment-resistant OCD, intensive daily CBT in a partial hospitalization or inpatient setting that is a tailor-made, patient-specific program is one of the most effective treatments, with response rates of up to 70%4-8 CBT’s advantages over medication include lower relapse rates and no known adverse effects. Unfortunately, CBT is underused9-11 due in part to a shortage of trained clinicians, and because patients may favor the ease of taking medication over the time, effort, and cost involved in CBT.
First-line pharmacologic options for treating OCD are SSRIs and clomipramine, as supported by multiple randomized controlled trials (RCTs), meta-analyses, expert guidelines, and consensus statements (Table 22,12-14). No significant difference has been found among SSRIs for the treatment of OCD in a review of 17 studies that included more than 3,000 patients.15 Treatment with SSRIs or clomipramine is effective for 50% to 60% of patients.16 Many clinicians view the combination of an SSRI and CBT as the treatment of choice for OCD.2

Continue to: Reluctance to engage in CBT
CASE CONTINUED
Reluctance to engage in CBT
To determine the next course of action, you review Mr. S’s treatment history. He has received adequate doses of 2 SSRIs and currently is taking clomipramine, 100 mg twice daily. He recently began CBT, which includes homework to help face his fears; however, Mr. S is reluctant to complete the exposure assignments, and after pausing for a few seconds as he tries to resist sending an apology email to his coworkers, he then returns to his compulsive behavior.
Facing treatment resistance
Although currently there isn’t a cure to resolve all traces of OCD, the goal of treatment is to decrease distress, interference, and the frequency of symptoms to a minimal level such that only the patients themselves are aware of symptoms. In broad terms, “response” has been defined as a decrease in symptoms, and “remission” has been defined as minimal symptoms after treatment.
Close to half of adults treated for OCD respond well to standard-of-care treatment (CBT and/or an SSRI), while the other 50% are considered partial responders or nonresponders.2 For patients with OCD, researchers often define “treatment response” as a ≥25% reduction in symptom severity score on the Y-BOCS. Approximately 30% of adults with OCD do not respond substantially to the first-line treatments, and even those who are defined as “responders” in research studies typically continue to have significant symptoms that impact their quality of life.2 In children, a clinical definition for treatment-refractory OCD has been presented as failing to achieve adequate symptom relief despite receiving an adequate course of CBT and at least 2 adequate trials of an SSRI or clomipramine.17 In the Pediatric OCD Treatment Study (POTS) trial, >46% of youth did not achieve remission from their OCD symptoms, even after receiving evidence-based care provided by experienced clinicians (combined treatment with CBT and an SSRI).18
_
Challenges in psychotherapy
Compassion is a key element in developing rapport with patients to help them face increasingly more challenging exposures. Making OCD the problem, not the person, is an essential element in helping patients move forward. Some clinicians may become frustrated with patients when treatment is not moving along well, referring to resistance, denial, or sabotage. According to March and Mulle,19 these terms lack the recognition and compassion that exposures are inherently difficult.19
Another challenge for therapists is if the patient’s presenting symptoms are personally offensive or a sensitive topic. For example, a therapist who is disgusted by public restrooms will find it difficult to tolerate the risks associated with exposure to germs and support a patient in touching objects in the restroom. Therapists also may be challenged when the patient’s fears align with the therapist’s religious beliefs. In these situations, consider transferring care to another therapist.
Family members need to learn about the nature of the illness and their roles in helping patients improve. Family members may unknowingly enable symptoms or criticize patients for their lack of motivation, which can lead to conflict in the home. Family dysfunction can in turn worsen OCD symptoms.
The most likely cause of lack of response to therapy is inexpert CBT.19 Deep breathing and relaxation training have been used as an active placebo in studies20; in a meta-analysis examining the effective components of CBT, studies that added relaxation training were not more effective than those that employed exposures alone.21 Patients receiving CBT should be able to articulate the hierarchical approach used to gradually face their fears.
Continue to: Pharmacologic augmentation strategies
Pharmacologic augmentation strategies
Selective serotonin reuptake inhibitors. While most OCD research trials have assessed SSRIs in 12-week studies, clinicians may consider extending SSRI treatment for an additional 12 weeks for nonresponders because some patients will continue to make gains. In the past, it was generally believed that higher doses of SSRIs are needed for treating OCD than for treating major depressive disorder. For instance, greater improvement was seen with 250 to 400 mg/d of sertraline compared with 200 mg/d22 and with escitalopram after an increase of dose up to 50 mg/d.23 However, more recently, this notion of higher doses being necessary for treatment response has been called into question. For example, a study of escitalopram found similar responses to 10 mg/d vs 20 mg/d after 24 weeks.24 A meta-analysis of adult studies of SSRIs for OCD supported higher doses as being more effective, but noted that the drop-out rate from treatment was greater in patients treated with higher doses.25 As a note of caution, long-term, high-dose maintenance therapy increases the risk of adverse reactions.26
Following a failed treatment with a first SSRI, it remains debatable as to what ought to be the second pharmacologic treatment. Although clomipramine is often reserved for treatment after 2 failed trials of an SSRI due to its greater risk of adverse effects, in an open-label study, switching from an SSRI to clomipramine led to greater response than switching from one SSRI to another.27 On the other hand, while meta-analyses have reported greater treatment effect for oral clomipramine than for SSRIs, direct head-to-head comparisons have not supported this notion.28 To get the best of both worlds, some clinicians employ a strategy of combining clomipramine with an SSRI, while monitoring for adverse effects and interactions such as serotonin syndrome.29-31
Benzodiazepines. Although benzodiazepines are useful for brief treatment of an anxiety disorder (eg, for a person with a fear of heights who needs to take an airplane),32 they have not been shown to be effective for OCD33 or as augmentation to an SSRI.34
N-acetylcysteine (NAC). Two RCTs of adults with OCD who received adjunctive NAC, 3 g/d in divided doses, found no significant difference in the treatment arms by the conclusion of 16 weeks—either both groups improved, or both groups failed to improve.35,36 In a 10-week study of patients with moderate to severe OCD symptoms, NAC, 2 g/d, as augmentation to fluvoxamine, 200 mg/d, showed a significant time x interaction in the treatment group.37 No follow-up information is available, however.
In a multicenter RCT of NAC given to children and adolescents with OCD as augmentation to citalopram, symptoms decreased and the quality-of-life score improved, with a large treatment effect size in the NAC group.38 However, in a study aimed at examining NAC in youth with Tourette syndrome, OCD symptoms were measured as a secondary outcome and there was no benefit of NAC over placebo.39
Memantine. Four 8- to 12-week RCTs in adults with OCD favored adjunctive memantine, 20 mg/d, taken with an SSRI, over placebo.40-43 A small study suggests that patients with OCD may be more likely to respond to memantine than patients with generalized anxiety disorder.44 Case reports have noted that memantine has been beneficial for pediatric patients with refractory OCD.45
Continue to: Topiramate
Topiramate. Three 12-week RCTs examined topiramate augmentation at 100 to 400 mg/d in patients with OCD who had failed at least 1 previous trial of an SSRI. The earliest study was most encouraging: Y-BOCS scores decreased by 32% in the topiramate group but by only 2.4% in the placebo group.46 However, the other 2 studies found no difference in the final OCD symptom severity score between active treatment and placebo groups,47,48 and the use of topiramate, particularly at higher doses, was limited by its adverse effects.
Lamotrigine. Initially, lamotrigine augmentation of SSRIs in OCD did not appear to be helpful.49 More recently, several case studies reported that lamotrigine, 100 to 200 mg/d, added to paroxetine or clomipramine, resulted in dramatic improvement in Y-BOCS scores for patients with long-standing refractory symptoms.50,51 In a retrospective review of 22 patients who received augmentation with lamotrigine, 150 mg/d, 20 had a significant response; the mean decrease in Y-BOCS score was 67%.52 Finally, in a 16-week RCT, lamotrigine, 100 mg/d, added to an SSRI led to a significant decrease in both Y-BOCS score and depressive symptoms while also improving semantic fluency.53
Ketamine. Ketamine is drawing increased attention for its nearly instantaneous antidepressant effect that lasts for up to 2 weeks after a single infusion.54 In a study of 15 medication-free adults with continuous intrusive obsessions, 4 of 8 patients who received a single IV infusion of ketamine, 0.5 mg/kg, met the criteria for treatment response (>35% reduction in Y-BOCS score measured 1 week later); none of the patients who received a placebo infusion of saline met this criteria.55 A small open-label trial of 10 treatment-refractory patients found that an infusion of ketamine, 0.5 mg/kg, was beneficial for comorbid depression but had only a minimal effect on OCD symptoms measured 3 days post-infusion.56 A short-term follow-up on these patients revealed dysphoria in some responders.57
D-cycloserine. The idea of using a pharmacologic agent to increase the speed or efficacy of behavioral therapy is intriguing. Proof of concept was demonstrated in a study that found that giving D-cycloserine prior to computerized exposure therapy significantly improved clinical response in patients with acrophobia.58 However, using this approach to treating OCD netted mixed results; D-cycloserine was found to be most helpful during early stages of treatment.59,60
Table 3 outlines the mechanisms of action and common uses for NAC, memantine, ketamine, topiramate, lamotrigine, and D-cycloserine. Table 4 summarizes the literature on the efficacy of some of the augmentation strategies for treating OCD described in this article.

Continue to: Alternative strategies
Alternative strategies
Augmentation strategies with neuroleptics,61 transcranial magnetic stimulation,62 and deep brain stimulation63 have recently been reviewed. Space limitations preclude a comprehensive review of these strategies, but in a cross-sectional study of augmentation strategies in OCD, no difference was found in terms of symptom severity between those prescribed SSRI monotherapy or augmentation with neuroleptics, benzodiazepines, or antidepressants.64

CASE CONTINUED
Progress in CBT
Mr. S agrees to a trial of NAC as an augmentation strategy, but after 8 weeks of treatment with NAC, 600 mg twice daily, his Y-BOCS had declined by only 2 points. He also complains of nausea and does not want to increase the dose. You discontinue NAC and opt to further explore his reaction to CBT. Mr. S shares that he has been seeing his psychologist only once every 3 weeks because he does not want to miss work. You encourage him to increase to weekly CBT sessions, and you obtain his permission to contact his therapist and his family members. Fortunately, his therapist is highly qualified, but unfortunately, Mr. S’s father has been sending him multiple critical emails about not advancing at his job and for being “lazy” at work. You schedule a session with Mr. S and his father. Great progress is made after Mr. S and his father both share their frustrations and come to understand and appreciate each other’s struggles. Four weeks later, after weekly CBT appointments, Mr. S has a Y-BOCS of 18 and spends <2 hours/d checking emails for errors and apologizing.
Bottom Line
It is unrealistic to expect OCD symptoms to be cured. Many ‘treatment-resistant’ patients have not received properly delivered cognitive-behavioral therapy, and this first-line treatment modality should be considered in every eligible patient, and augmented with a selective serotonin reuptake inhibitor (SSRI) when needed. Glutamatergic agents, in turn, can augment SSRIs.
Related Resources
- Yale-Brown Obsessive-Compulsive Scale. https://iocdf.org/ wp-content/uploads/2014/08/Assessment-Tools.pdf.
- The International OCD Foundation. https://iocdf.org.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Ketamine • Ketalar
Lamotrigine • Lamictal
Memantine • Namenda
Paroxetine • Paxil
Sertraline • Zoloft
Topiramate • Topomax
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry; 2007;164(suppl 7):5-53.
3. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
4. Bystritsky A, Munford PR, Rosen RM, et al. A preliminary study of partial hospital management of severe obsessive-compulsive disorder. Psychiatr Serv. 1996;47(2):170-174.
5. Calvocoressi L, McDougle CI, Wasylink S, et al. Inpatient treatment of patients with severe obsessive-compulsive disorder. Hosp Community Psychiatry. 1993;44(12):1150-1154.
6. Eddy KT, Dutra L, Bradley R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
7. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
8. Simpson HB, Huppert JD, Petkova E, et al. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(2):269-276.
9. Marques L, LeBlanc NJ, Weingarden HM, et al. Barriers to treatment and service utilization in an internet sample of individuals with obsessive-compulsive symptoms. Depress Anxiety. 2010;27(5):470-475.
10. Goodwin R, Koenen KC, Hellman F, et al. Helpseeking and access to mental health treatment for obsessive-compulsive disorder. Acta Psychiatr Scand. 2002;106(2):143-149.
11. Kohn R, Saxena S, Levav I, et al. The treatment gap in mental health care. Bull World Health Organ. 2004;82(11):858-866.
12. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439.
13. Lovell K, Bee P. Implementing the NICE OCD/BDD guidelines. Psychol Psychother. 2008;81(Pt 4):365-376.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84.
15. Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;(1):CD001765.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
17. Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in children. J Am Acad Child Adolesc Psychiatry. 2015;54(4):251-262.
18. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
19. March JS, Mulle K. OCD in children and adolescents: a cognitive-behavioral treatment manual. New York, NY: Guilford Press; 1998.
20. Marks IM. Fears, phobias, and rituals: Panic, anxiety, and their disorders. 1987, New York, NY: Oxford University Press; 1987.
21. Ale CM, McCarthy DM, Rothschild LM, et al. Components of cognitive behavioral therapy related to outcome in childhood anxiety disorders. Clin Child Fam Psychol Rev. 2015;18(3):240-251.
22. Ninan PT, Koran LM, Kiev A, et al. High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. J Clin Psychiatry. 2006;67(1):15-22.
23. Rabinowitz I, Baruch Y, Barak Y. High-dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2008;23(1):49-53.
24. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
25. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
26. Sayyah M, Majzoob S, Sayyah M. Metabolic and toxicological considerations for obsessive-compulsive disorder drug therapy. Expert Opin Drug Metab Toxicol. 2013;9(6):657-673.
27. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry. 2002;63(suppl 6):20-29.
28. Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005;8(1):107-129.
29. Marazziti D, Golia F, Consoli G, et al. Effectiveness of long-term augmentation with citalopram to clomipramine in treatment-resistant OCD patients. CNS Spectr. 2008;13(11):971-976.
30. Browne M, Horn E, Jones TT. The benefits of clomipramine-fluoxetine combination in obsessive compulsive disorder. Can J Psychiatry. 1993;38(4):242-243.
31. Ravizza L, Barzega G, Bellino S, et al. Drug treatment of obsessive-compulsive disorder (OCD): long-term trial with clomipramine and selective serotonin reuptake inhibitors (SSRIs). Psychopharmacol Bull. 1996;32(1):167-173.
32. Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci. 2011;13(4):423-437.
33. Hollander E, Kaplan A, Stahl SM. A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J Biol Psychiatry. 2003;4(1):30-34.
34. Crockett BA, Churchill E, Davidson JR. A double-blind combination study of clonazepam with sertraline in obsessive-compulsive disorder. Ann Clin Psychiatry. 2004;16(3):127-132.
35. Costa DLC, Diniz JB, Requena G, et al. Randomized, double-blind, placebo-controlled trial of n-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7):e766-e773.
36. Sarris J, Oliver G, Camfield DA, et al. N-Acetyl Cysteine (NAC) in the treatment of obsessive-compulsive disorder: a 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29(9):801-809.
37. Paydary K, Akamaloo A, Ahmadipour A, et al. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016;41(2):214-219.
38. Ghanizadeh A, Mohammadi MR, Bahraini S, et al. Efficacy of N-acetylcysteine augmentation on obsessive compulsive disorder: a multicenter randomized double blind placebo controlled clinical trial. Iran J Psychiatry. 2017;12(2):134-141.
39. Bloch MH, Panza KE, Yaffa A, et al. N-acetylcysteine in the treatment of pediatric tourette syndrome: randomized, double-blind, placebo-controlled add-on trial. J Child Adolesc Psychopharmacol. 2016;26(4):327-334.
40. Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47(2):175-180.
41. Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34-39.
42. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2017. doi: 10.1055/s-0043-120268. [Epub ahead of print].
43. Haghighi M, Jahangard L, Mohammad-Beigi H, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013;228(4):633-640.
44. Feusner JD, Kerwin L, Saxena S, et al. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81-93.
45. Hezel DM, Beattie K, Stewart SE. Memantine as an augmenting agent for severe pediatric OCD. Am J Psychiatry. 2009;166(2):237.
46. Mowla A, Khajeian AM, Sahraian A, et al. topiramate augmentation in resistant ocd: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15(11):613-617.
47. Berlin H, Koran LM, Jenike MA, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(5):716-721.
48. Afshar H, Akuchekian S, Mahaky B, et al. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(10):976-981.
49. Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry. 2000;34(3):527-528.
50. Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry. 2013;2013:612459.
51. Uzun O. Lamotrigine as an augmentation agent in treatment-resistant obsessive-compulsive disorder: a case report. J Psychopharmacol. 2010;24(3):425-427.
52. Hussain A, Dar MA, Wani RA, et al. Role of lamotrigine augmentation in treatment-resistant obsessive compulsive disorder: a retrospective case review from South Asia. Indian J Psychol Med. 2015;37(2):154-158.
53. Bruno A, Micò U, Pandolfo G, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol. 2012;26(11):1456-1462.
54. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73(12):113311-41.
55. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483.
56. Bloch MH, Wasylink S, Landeros-Weisenberger A,, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964-970.
57. Niciu MJ, Grunschel BD, Corlett PR, et al. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
58. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136-1144.
59. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
60. Xia J, Du Y, Han J, et al. D-cycloserine augmentation in behavioral therapy for obsessive-compulsive disorder: a meta-analysis. Drug Des Devel Ther. 2015;9:2101-2117.
61. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:317.
62. Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. 2017;33(6):747-756.
63. Naesström, M, Blomstedt P, Bodlund O. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Nord J Psychiatry. 2016;70(7):483-491.
64. Van Ameringen M, Simpson W, Patterson B, et al. Pharmacological treatment strategies in obsessive compulsive disorder: A cross-sectional view in nine international OCD centers. J Psychopharmacol, 2014;28(6):596-602.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry; 2007;164(suppl 7):5-53.
3. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
4. Bystritsky A, Munford PR, Rosen RM, et al. A preliminary study of partial hospital management of severe obsessive-compulsive disorder. Psychiatr Serv. 1996;47(2):170-174.
5. Calvocoressi L, McDougle CI, Wasylink S, et al. Inpatient treatment of patients with severe obsessive-compulsive disorder. Hosp Community Psychiatry. 1993;44(12):1150-1154.
6. Eddy KT, Dutra L, Bradley R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011-1030.
7. Abramowitz JS. The psychological treatment of obsessive-compulsive disorder. Can J Psychiatry. 2006;51(7):407-416.
8. Simpson HB, Huppert JD, Petkova E, et al. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(2):269-276.
9. Marques L, LeBlanc NJ, Weingarden HM, et al. Barriers to treatment and service utilization in an internet sample of individuals with obsessive-compulsive symptoms. Depress Anxiety. 2010;27(5):470-475.
10. Goodwin R, Koenen KC, Hellman F, et al. Helpseeking and access to mental health treatment for obsessive-compulsive disorder. Acta Psychiatr Scand. 2002;106(2):143-149.
11. Kohn R, Saxena S, Levav I, et al. The treatment gap in mental health care. Bull World Health Organ. 2004;82(11):858-866.
12. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439.
13. Lovell K, Bee P. Implementing the NICE OCD/BDD guidelines. Psychol Psychother. 2008;81(Pt 4):365-376.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84.
15. Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;(1):CD001765.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375-391.
17. Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive-compulsive disorder in children. J Am Acad Child Adolesc Psychiatry. 2015;54(4):251-262.
18. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
19. March JS, Mulle K. OCD in children and adolescents: a cognitive-behavioral treatment manual. New York, NY: Guilford Press; 1998.
20. Marks IM. Fears, phobias, and rituals: Panic, anxiety, and their disorders. 1987, New York, NY: Oxford University Press; 1987.
21. Ale CM, McCarthy DM, Rothschild LM, et al. Components of cognitive behavioral therapy related to outcome in childhood anxiety disorders. Clin Child Fam Psychol Rev. 2015;18(3):240-251.
22. Ninan PT, Koran LM, Kiev A, et al. High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. J Clin Psychiatry. 2006;67(1):15-22.
23. Rabinowitz I, Baruch Y, Barak Y. High-dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2008;23(1):49-53.
24. Stein DJ, Andersen EW, Tonnoir B, et al. Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin. 2007;23(4):701-711.
25. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
26. Sayyah M, Majzoob S, Sayyah M. Metabolic and toxicological considerations for obsessive-compulsive disorder drug therapy. Expert Opin Drug Metab Toxicol. 2013;9(6):657-673.
27. Hollander E, Bienstock CA, Koran LM, et al. Refractory obsessive-compulsive disorder: state-of-the-art treatment. J Clin Psychiatry. 2002;63(suppl 6):20-29.
28. Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005;8(1):107-129.
29. Marazziti D, Golia F, Consoli G, et al. Effectiveness of long-term augmentation with citalopram to clomipramine in treatment-resistant OCD patients. CNS Spectr. 2008;13(11):971-976.
30. Browne M, Horn E, Jones TT. The benefits of clomipramine-fluoxetine combination in obsessive compulsive disorder. Can J Psychiatry. 1993;38(4):242-243.
31. Ravizza L, Barzega G, Bellino S, et al. Drug treatment of obsessive-compulsive disorder (OCD): long-term trial with clomipramine and selective serotonin reuptake inhibitors (SSRIs). Psychopharmacol Bull. 1996;32(1):167-173.
32. Koen N, Stein DJ. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci. 2011;13(4):423-437.
33. Hollander E, Kaplan A, Stahl SM. A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J Biol Psychiatry. 2003;4(1):30-34.
34. Crockett BA, Churchill E, Davidson JR. A double-blind combination study of clonazepam with sertraline in obsessive-compulsive disorder. Ann Clin Psychiatry. 2004;16(3):127-132.
35. Costa DLC, Diniz JB, Requena G, et al. Randomized, double-blind, placebo-controlled trial of n-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78(7):e766-e773.
36. Sarris J, Oliver G, Camfield DA, et al. N-Acetyl Cysteine (NAC) in the treatment of obsessive-compulsive disorder: a 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29(9):801-809.
37. Paydary K, Akamaloo A, Ahmadipour A, et al. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016;41(2):214-219.
38. Ghanizadeh A, Mohammadi MR, Bahraini S, et al. Efficacy of N-acetylcysteine augmentation on obsessive compulsive disorder: a multicenter randomized double blind placebo controlled clinical trial. Iran J Psychiatry. 2017;12(2):134-141.
39. Bloch MH, Panza KE, Yaffa A, et al. N-acetylcysteine in the treatment of pediatric tourette syndrome: randomized, double-blind, placebo-controlled add-on trial. J Child Adolesc Psychopharmacol. 2016;26(4):327-334.
40. Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47(2):175-180.
41. Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34-39.
42. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2017. doi: 10.1055/s-0043-120268. [Epub ahead of print].
43. Haghighi M, Jahangard L, Mohammad-Beigi H, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013;228(4):633-640.
44. Feusner JD, Kerwin L, Saxena S, et al. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81-93.
45. Hezel DM, Beattie K, Stewart SE. Memantine as an augmenting agent for severe pediatric OCD. Am J Psychiatry. 2009;166(2):237.
46. Mowla A, Khajeian AM, Sahraian A, et al. topiramate augmentation in resistant ocd: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15(11):613-617.
47. Berlin H, Koran LM, Jenike MA, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(5):716-721.
48. Afshar H, Akuchekian S, Mahaky B, et al. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(10):976-981.
49. Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry. 2000;34(3):527-528.
50. Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry. 2013;2013:612459.
51. Uzun O. Lamotrigine as an augmentation agent in treatment-resistant obsessive-compulsive disorder: a case report. J Psychopharmacol. 2010;24(3):425-427.
52. Hussain A, Dar MA, Wani RA, et al. Role of lamotrigine augmentation in treatment-resistant obsessive compulsive disorder: a retrospective case review from South Asia. Indian J Psychol Med. 2015;37(2):154-158.
53. Bruno A, Micò U, Pandolfo G, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol. 2012;26(11):1456-1462.
54. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73(12):113311-41.
55. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483.
56. Bloch MH, Wasylink S, Landeros-Weisenberger A,, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964-970.
57. Niciu MJ, Grunschel BD, Corlett PR, et al. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013;27(7):651-654.
58. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136-1144.
59. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
60. Xia J, Du Y, Han J, et al. D-cycloserine augmentation in behavioral therapy for obsessive-compulsive disorder: a meta-analysis. Drug Des Devel Ther. 2015;9:2101-2117.
61. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:317.
62. Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. 2017;33(6):747-756.
63. Naesström, M, Blomstedt P, Bodlund O. A systematic review of psychiatric indications for deep brain stimulation, with focus on major depressive and obsessive-compulsive disorder. Nord J Psychiatry. 2016;70(7):483-491.
64. Van Ameringen M, Simpson W, Patterson B, et al. Pharmacological treatment strategies in obsessive compulsive disorder: A cross-sectional view in nine international OCD centers. J Psychopharmacol, 2014;28(6):596-602.
Children with tic disorders: How to match treatment with symptoms
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Sammy, age 7, is referred to you by his pediatrician because of a 4-week history of frequent eye blinking. His parents say he blinks a lot when bored but very little when playing baseball. They recall that he also has intermittently sniffed and nodded his head over the last 12 months. Neither Sammy nor his friends seem to be bothered by the blinking. Except for the tics, Sammy’s physical and mental status exams are normal.
Since preschool, Sammy’s teachers have complained that his backpack and desk are always a mess. Sammy is well-meaning but forgetful in his chores at home. A paternal uncle has head-turning movements, counts his steps, and becomes distressed if books on his shelf are not in alphabetical order.
Tics, such as strong eye blinks or repetitive shoulder shrugs, can distress a child or his/her parents, but the conditions associated with tic disorders often are more problematic than the tic disorder itself. High rates of comorbid conditions are recognized in persons with Tourette syndrome, including:
- obsessive-compulsive disorder (OCD) in >80%1
- attention-deficit/hyperactivity disorder (ADHD) in ≤70%2
- anxiety disorders in 30%3
- rage, aggression, learning disabilities, and autism less commonly.
The strategy we recommend for managing tic disorders includes assessing tic severity, educating the family about the illness, determining whether a comorbid condition is present, and managing these conditions appropriately. Above all, we emphasize a risk-benefit analysis guided by the Hippocratic principle of “do no harm.”
Characteristics of tic disorders
You diagnose Sammy with Tourette syndrome because he meets DSM-IV-TR criteria of at least 2 motor tics and 1 vocal tic that have persisted for 1 year without more than a 3-month hiatus, with tic onset before age 18. Because tics may resemble other movement disorders, you rule out stereotypies, dystonia, chorea, ballism, and myoclonus (Table 1). You explain to his parents that Sammy’s condition is a heritable, neurobehavioral disorder that typically begins in childhood and is associated in families with OCD, ADHD, and autism spectrum disorders.
His parents ask about the difference between tics and other movements. You explain that eye-blinking tics—like other motor tics—appear as sudden, repetitive, stereotyped, nonrhythmic movements that involve discrete muscle groups. (View a video of a patient with tics.) Simple motor tics are focal movements involving 1 group of muscles, whereas complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements (Table 2).
Table 1
Features of 5 movement disorders that may resemble tics
| Tics | Stereotypies | Dystonia | Chorea | Ballism | Myoclonus |
|---|---|---|---|---|---|
| Sudden, repetitive, stereotyped, nonrhythmic movements or sounds | Patterned, nonpurposeful movement | Cocontraction of agonist and antagonist muscles, causing an abnormal twisting posture | Continuous, flowing, nonrhythmic, nonpurposeful movement | Forceful, flinging, large amplitude choreic movement | Sudden, quick, shock-like movement |
| Usually start after age 3 | Usually start before age 3 and resolve by adolescence | More common in adults | — | — | — |
| Decrease when focused; increase when stressed, anxious, fatigued, or bored | Occur when the child is excited | Worsens during motor tasks | Worsens during motor tasks | Worsens during motor tasks | — |
| Comorbid conditions include OCD and ADHD | Common in children with mental retardation or autism | — | Can occur after streptococcal infection | Can occur after streptococcal infection | — |
| Preceded by a premonitory urge or sensation | Possibly preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge | Not preceded by an urge |
| Temporarily suppressible | Suppressible | Not suppressible | Partially suppressible; can incorporate into semi-purposeful movements | Partially suppressible | Not suppressible |
| ADHD: attention-deficit/hyperactivity disorder; OCD: obsessive-compulsive disorder | |||||
Table 2
Characteristics of simple and complex motor and vocal tics*
| Simple tics | Complex tics |
|---|---|
| Eye blinking or eye rolling Nose, mouth, tongue, or facial grimaces (nose twitch, nasal flaring, chewing lip, teeth grinding, sticking out tongue, mouth stretching, lip licking) Head jerks or movements (neck stretching, touching chin to shoulder) Shoulder jerks/movements (shoulder shrugging, jerking a shoulder) Arm or hand movements (flexing or extending arms or fingers) Coughing Throat clearing, grunting Sniffing, snorting, shouting Humming | Jumping Spinning Touching objects or people Throwing objects Repeating others’ action (echopraxia) Obscene gestures (copropraxia) Repeating one’s own words (palilalia) Repeating what someone else said (echolalia) Obscene, inappropriate words (coprolalia) |
| *Simple tics are focal movements involving 1 group of muscles; complex tics are sequential patterns of movement that involve >1 muscle group or resemble purposeful movements | |
Older children frequently describe a premonitory urge prior to the tic. Patients typically can suppress tics for a transient period of time, although during tic suppression they usually feel restless and anticipate performing their tic. The ultimate performance of the tic brings relief. Tic suppression also occurs during focused activity. Emotional stress, fatigue, illness, or boredom can exacerbate tics.
To begin monitoring Sammy’s clinical course, you administer 3 assessment tools described inTable 3. You explain to Sammy’s parents that these tests will be repeated yearly or when tics worsen. However, you tell his parents that these scores alone will not determine present or future clinical decisions, including treatments. You also recommend that they connect with support groups on the Tourette Syndrome Association (TSA) Web site.
CASE CONTINUED: Changes over time
Sammy’s parents appreciate your explanation and say they will share information from the TSA Web site with Sammy’s principal, teachers, and classmates. The family agrees to return in 6 months or sooner if the tics worsen.
By age 8, Sammy develops multiple tics: facial grimacing, looking upwards, punching movements, whistling, and throat clearing. He is slightly bothered by these tics, and his friends have asked him about them. He tells them he has Tourette syndrome, and that usually ends the questioning. He returns for a follow-up visit because his parents notice a dramatic increase in his tics after Sammy’s father loses his job.
Treatment options
When deciding to treat a child’s tics, the first step is to determine whether to pursue a nonpharmacologic or pharmacologic approach (Algorithm). To tailor an approach most suited for an individual child, discuss with the family their feelings about therapy and medications. This information—along with tic severity—will help determine a treatment plan.
Behavior therapy and medication are management strategies; neither can cure a tic disorder. The most conservative approach to tic treatment is to:
- provide the child and family with basic guidelines for managing tics
- help alleviate environmental stress and other potential triggers.
Algorithm: Recommended treatment of tics in children and adolescents
CASE CONTINUED: A first intervention
You discuss treatment options with Sammy’s family, and they view medication as a last resort. Sammy does not seem to be bothered by his tics, and his parents do not wish to start him on daily medications. Given this situation, habit reversal therapy (HRT) is appropriate for Sammy because he is old enough to participate in HRT to reduce his tics.
HRT is an effective nonpharmacologic approach to help children with tics.4 Its 3 components are:
- awareness training
- competing response training
- social support.5
This simplified version of the original HRT can be completed in eight 1-hour sessions. Good candidates are patients who are cognitively mature enough to understand the therapy’s goals and compliant with frequent clinic visits. They also must practice the strategies at home.
It should not be difficult for psychiatrists to learn HRT—or refer to therapists who are willing to learn it—with the available instructional manual.
CASE CONTINUED: Practicing alternatives
You ask Sammy to imitate his tics. After helping him become more aware of his tics, you encourage him to develop a more socially appropriate movement to engage in whenever he feels the urge to punch. Sammy chooses to clench his fist in his pocket. He also learns to breathe in whenever he has an urge to whistle. you advise Sammy’s parents to reward his efforts to suppress the tics. He practices the strategies daily.
At age 12, Sammy returns to your office. He has begun to have frequent neck-jerking tics, which cause neck pain and daily headaches. He also is slapping his thigh and having frequent vocal tics characterized by loud shrieking. The vocal tics are disruptive in class, even though Sammy sits toward the back of the room. Sammy’s classmates tease him, and he is very frustrated.
Medication approach
The decision to start a medication for tics is complex. Scores from the YGTSS, PUTS, and GTS-QOL scales (Table 3) provide only a partial clinical picture. This decision should be reached after a detailed discussion with the family about benefits and risks of medications and ensuring that everyone’s expectations are reasonable.
A variety of medications are available to treat patients with tics (Table 4). No medication can completely eliminate tics, however, and many have substantial side effects. Before initiating medical treatment, consider 3 questions:
- Is moderate or severe pain involved?
- Is there significant functional interference?
- Is there significant social disruption despite efforts to optimize the social environment for the child?
Sammy’s frequent neck-jerking tics now cause chronic daily headaches, and his shrieking vocal tics are interfering with classroom activities, so we recommended a 3-month trial of guanfacine following the dosing schedule in Table 4.
Table 3
3 scales for assessing tic severity and impact on functioning
| Instrument | Purpose | Description | Design | Administration frequency |
|---|---|---|---|---|
| Yale Global Tic Severity Scale (YGTSS) | Assess tic severity | Review of motor and vocal tics. Rate number, frequency, intensity, complexity, and interference on a 5-point scale | Clinician-rated | Annual and as needed for increased tics |
| Premonitory Urge for Tics Scale (PUTS) | Detect the presence of unpleasant sensations that precedes tics | 10 questions | Self-report | Annual and as needed for increased tics |
| Gilles de la Tourette Syndrome Quality of Life Scale (GTS-QOL) | Measure quality of life | 27 questions, 4 subscales: psychological, physical, obsessional, and cognitive | Self-report | Annual and as needed for increased tics |
Table 4
Medications with evidence of tic-suppressing effects*
| Category A evidence | ||
|---|---|---|
| Medication | Starting dose | Target dose |
| Haloperidol | 0.25 to 0.5 mg/d | 1 to 4 mg/d |
| Pimozide | 0.5 to 1 mg/d | 2 to 8 mg/d |
| Risperidone | 0.25 to 0.5 mg/d | 1 to 3 mg/d |
| Category B evidence | ||
| Medication | Starting dose | Target dose |
| Fluphenazine | 0.5 to 1 mg/d | 1.5 to 10 mg/d |
| Ziprasidone | 5 to 10 mg/d | 10 to 80 mg/d |
| Clonidine | 0.025 to 0.05 mg/d | 0.1 to 0.3 mg/d |
| Guanfacine | 0.5 to 1 mg/d | 1 to 3 mg/d |
| Botulinum toxin | 30 to 300 units | |
| Category C evidence | ||
| Medication | Starting dose | Target dose |
| Olanzapine | 2.5 to 5 mg/d | 2.5 to 12.5 mg/d |
| Tetrabenazine | 25 mg/d | 37.5 to 150 mg/d |
| Baclofen | 10 mg/d | 40 to 60 mg/d |
| Nicotine patch | 7 mg/d | 7 to 21 mg/d |
| Mecamylamine | 2.5 mg/d | 2.5 to 7.5 mg/d |
| Flutamide | 250 mg/d | 750 mg/d |
| *Category A: supported by ≥2 placebo-controlled trials; category B: supported by 1 placebo-controlled trial; category C: supported by open-label study | ||
| Source: Reference 6 | ||
The first-line pharmacologic agent for tic suppression generally is an alpha-adrenergic medication, unless the tics are severe.6
Clonidine and guanfacine usually are started at low doses and increased gradually. Although not as effective as neuroleptics, alpha-adrenergics have a lower potential for side effects and are easier to use because no laboratory tests need to be monitored. Adverse effects associated with alpha-adrenergic medications include sedation, dry mouth, dizziness, headache, and rebound hypertension if discontinued abruptly.
If tics are causing pain, some clinicians prefer conservative measures such as heat or ice, massage, analgesics, relaxation therapy, and reassurance.
Second-line agents include typical and atypical antipsychotics. Haloperidol and pimozide have shown efficacy in reducing tics in placebo- controlled studies,7,8 as have risperidone (in 4 randomized controlled trials [RCTs]) and ziprasidone (in 1 RCT).9,10 The emergence of serious side effects is a risk for both typical and atypical antipsychotics (Table 5).
Table 5
Potential adverse effects of antipsychotic treatment in children*
| Adverse effect | Examples |
|---|---|
| Sedation | — |
| Acute dystonic reactions | Oculogyric crisis, torticollis |
| Appetite changes | Weight gain |
| Endocrine abnormalities | Amenorrhea, diabetes, galactorrhea, gynecomastia, hyperprolactinemia |
| Cognitive effects | Impaired concentration |
| Akathisia | Difficulty sitting still |
| ECG changes | Prolonged QT interval |
| Parkinsonism | Tremor, bradykinesia, rigidity, postural instability |
| Tardive syndrome | Orofacial dyskinesia, chorea, dystonia, myoclonus, tics |
| Neuroleptic malignant syndrome | Potentially fatal; consists of muscular rigidity, fever, autonomic dysfunction, labile blood pressure, sweating, urinary incontinence, fluctuating level of consciousness, leukocytosis, elevated serum creatine kinase |
| *Potential adverse effects are listed from most to least likely to occur | |
As part of your informed consent discussion, weigh the risk of side effects against the benefits of treatment. Point out to patients and their families that they can expect to see a decrease in tic frequency, but symptoms will not necessarily disappear with any medication. We tell our patients that with antipsychotics the best we can hope for is to reduce tic frequency by approximately one-half.6
When treating tics, start with 1 medication. However, if the tics are severe enough to require more than 1 medication, check for drug interactions.
Third-line agents. Agents that have not been tested in placebo-controlled trials can be considered third line; these are listed as category C (supported by open-label studies) in Table 4. Botulinum toxin injection has been found to be effective for motor and vocal tics.11,12 Botulinum toxin and implantation of deep brain stimulators13 are invasive options and generally are reserved for severe, treatment-resistant tics.
CASE CONTINUED: Managing antipsychotics
After trying guanfacine for 12 weeks, Sammy notices no tic reduction. His parents consent to a low dose of risperidone. you review with them the American Psychiatric Association (APA)/American Diabetes Association (ADA) guidelines14 for managing metabolic problems in patients treated with atypical antipsychotics.
As instructed in the APA/ADA guidelines, obtain baseline measurements and monitor for metabolic effects of antipsychotic therapy over time (Table 6). Sammy starts risperidone at 0.5 mg once daily. After 2 weeks, he notices a decrease in his tics. At the 3-month visit after starting risperidone, he is happy with his risperidone dose and does not want to increase it. He has gained 3 pounds, and you instruct him to eat a well-balanced diet and exercise routinely. At the 6-month visit, his tics are minimal and his weight has stabilized.
Table 6
Children receiving antipsychotics: monitoring recommendations
| Clinical information | Frequency |
|---|---|
| Family history | Initial visit |
| Weight | Baseline, monthly |
| Height | Baseline, monthly |
| BMI | Baseline, monthly |
| Waist circumference | Baseline, annually |
| Blood pressure | Baseline, 3 months after treatment starts, and annually thereafter |
| Fasting lipid profile | Baseline, every 3 months initially, then every 6 months thereafter |
| Fasting serum glucose | Baseline, every 3 months, then every 6 months thereafter |
| BMI: body mass index | |
| Source: References 14,16 | |
You recommend that Sammy remain on risperidone for another 3 months of stability and then begin to taper this medication. You review the risks and benefits of long-term treatment with risperidone, pointing out that it may lead to abnormal movements upon withdrawal, and explain that you typically do not treat children with antipsychotics for more than one year continuously.
CASE CONTINUED: Comorbid symptoms
Since starting 7th grade, Sammy has worried excessively about making mistakes. He spends 6 hours each night on homework, which he often does not turn in because of anxiety about not getting answers perfectly right. Classmates notice that Sammy taps the door 3 times when he comes into the classroom and that he steps over the black tiles in the hallway.
Consider the presence and impact of comorbid OCD or ADHD, which can impair children’s quality of life more than tics themselves.15 Assessment scales can help you make a diagnosis and monitor treatment.
If you suspect OCD, the clinician-rated Children’s Yale Brown Obsessive Compulsive Scale is the gold standard for describing the phenomenology and measuring symptom severity. Additional scales to measure symptoms’ impact on family life include the Leyton Obsessional Inventory—child version, Family Accommodation Scale for OCD, and Child OCD Impact Scale.
ADHD scales include the Conners Parent Rating Scale—Revised, Conners Teacher Rating Scale—Revised, Swanson, Nolan, and Pelham, or the Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales. Because ADHD symptoms must be present in more than 1 environment to meet diagnostic criteria, ask parents and teachers to complete the Conners or Vanderbilt scales.
In children who present with a tic disorder plus a comorbid condition, prioritize treatment by determining which symptoms interfere with the child’s ability to function at school, at home, and in the social arena. Children who require treatment for >1 disorder often are referred initially for cognitive-behavioral therapy for OCD symptoms while receiving pharmacologic treatment for ADHD and/or Tourette syndrome. When necessary, it is usually safe to combine antipsychotics, stimulants, and selective serotonin reuptake inhibitors, although medication interactions should be reviewed in each specific case.
Related resources
- Woods DW. Managing Tourette syndrome: a behavioral intervention for children and adults. Therapist guide. New York, NY: Oxford University Press; 2008.
- Tourette Syndrome Association. www.tsa-usa.org.
- International OCD Foundation. www.ocfoundation.org.
Drug brand names
- Baclofen • Lioresal
- Botulinum toxin • Botox, Myobloc
- Clomipramine • Anafranil
- Clonidine • Catapres
- Guanfacine • Tenex
- Fluphenazine • Prolixin
- Flutamide • Eulexin
- Haloperidol • Haldol
- Mecamylamine • Inversine
- Nicotine patch • NicoDerm
- Olanzapine • Zyprexa
- Pimozide • Orap
- Risperidone • Risperdal
- Tetrabenazine • Xenazine
- Ziprasidone • Geodon
Disclosures
Dr. Harris has received research support from the Translational Research Initiative at Cincinnati Children’s Hospital Medical Center.
Dr. Wu reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.
1. Robertson M. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(3):425-462.
2. Freeman R. For the Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a worldwide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(suppl 1):15-23.
3. Stefl M. Mental health needs associated with Tourette syndrome. Am J Public Health. 1984;74:1310-1313.
4. Deckersbach T, Rauch S, Buhlmann U, et al. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079-1090.
5. Woods DW, Miltenberger RG. Habit reversal: a review of applications and variations. J Behav Ther Exp Psychiatry. 1995;26:123-131.
6. Scahill L, Erenberg G, Berlin C, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
7. Shapiro E, Shapiro A, Fulop G, et al. Controlled study of haloperidol, pimozide, and placebo for the treatment of Gilles de la Tourette’s syndrome. 1989;46:722-730.
8. Sallee F, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154:1057-1062.
9. Scahill L, Leckman J, Schultz R, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130-1135.
10. Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
11. Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
12. Porta M, Maggioni G, Ottaviani F, et al. Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci. 2004;24(6):420-423.
13. Porta M, Sevello D, Sassi M, et al. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur Neurol. 2009;62(5):264-273.
14. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:1335-1342.
15. Bernard BA, Stebbins GT, Siegel S, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. 2009;24(7):1070-1073.
16. Understanding the risks of antipsychotic treatment in young people. Advice for managing side effects in children and teenagers. Harv Ment Health Lett. 2009;25(9):1-3.