User login
Serotonin syndrome: How to keep your patients safe
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
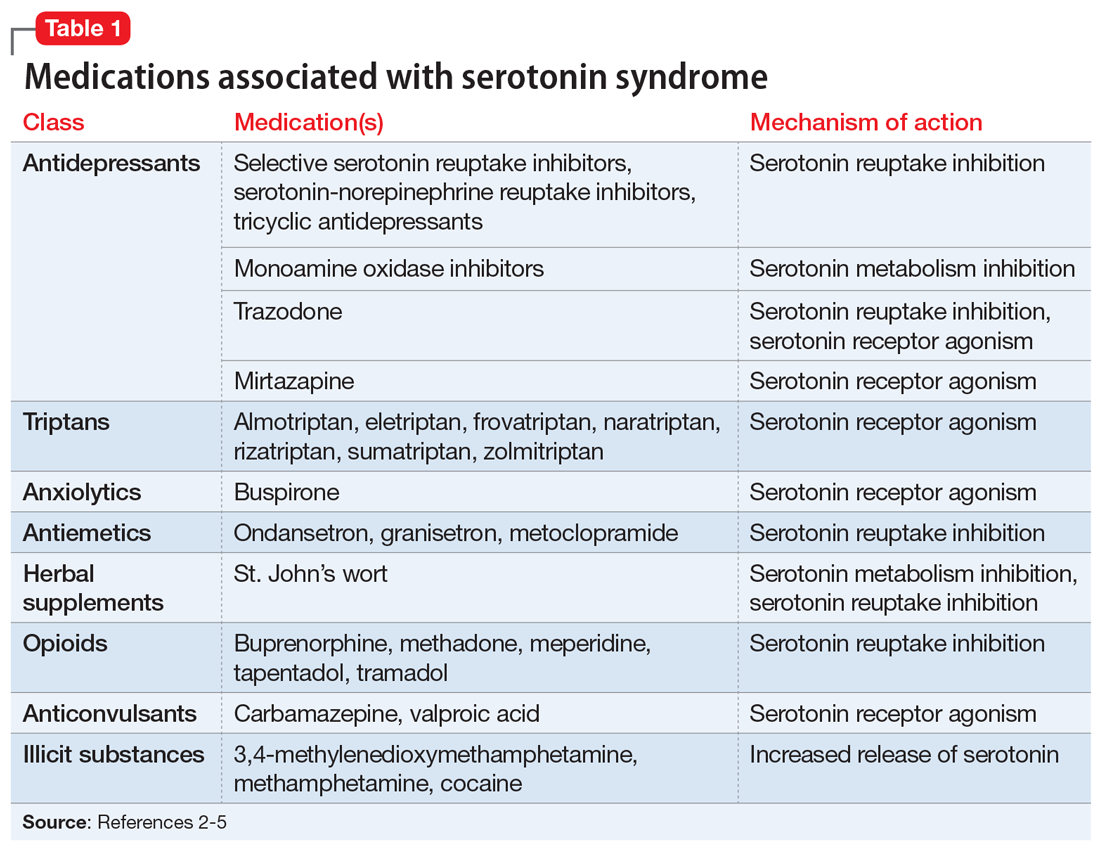
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
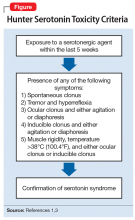
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
Treating psychosis in patients with HIV/AIDS
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.
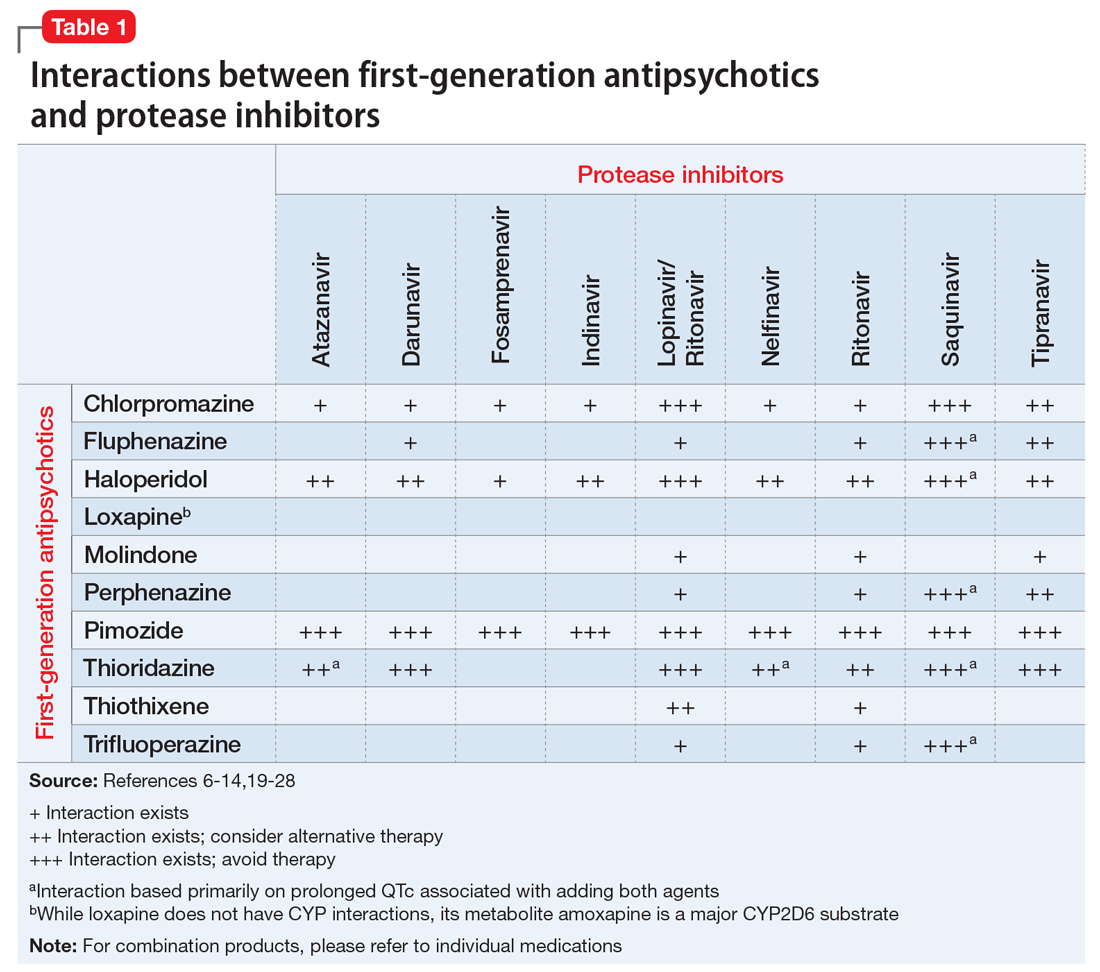
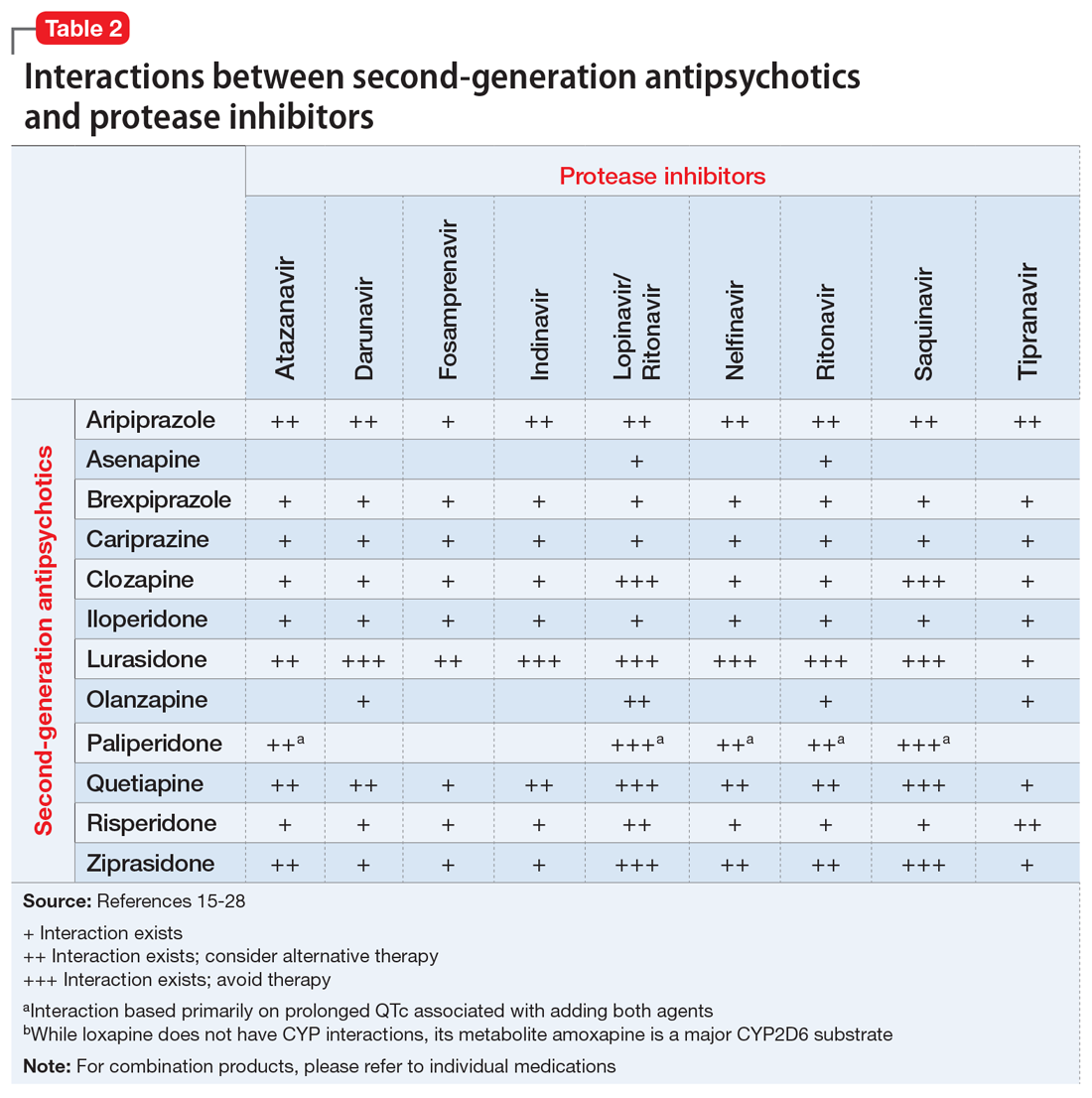
Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.
These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.
Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.
These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
Mr. S, age 56, has human immunodeficiency virus (HIV) and schizoaffective disorder. He presents to your clinic with increased auditory hallucinations, disorganized behavior, and worsened tremors that have begun to seriously disrupt his daily life. Mr. S is prescribed risperidone; however, he reports that he has not been taking it lately due to the tremor despite being controlled on his medication regimen for nearly 1 year. His Abnormal Involuntary Movement Scale (AIMS) score reveals an increased wrist rigidity compared with previous clinic visits. Mr. S has a 40 pack-year history of smoking and history of IV drug use. Furthermore, he has a medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia.
His medication regimen includes atazanavir sulfate, 200 mg/d, ritonavir, 100 mg/d, efavirenz/emtricitabine/tenofovir disoproxil fumarate, 600/200/300 mg/d, risperidone, 6 mg/d, bupropion extended-release, 300 mg/d, gabapentin, 600 mg/d, amlodipine, 5 mg/d, pravastatin, 40 mg/d, metformin, 1000 mg twice daily, and glipizide, 10 mg twice daily. Today, his laboratory findings show that his CD4 count is 405 cell/mm3, and his viral load is <40 copies/mL, indicating his HIV is well managed. A hepatitis C virus antibody test result is negative and serum creatinine level is 1.0 mg/dL. Total cholesterol is 212 mg/dL, high-density lipoprotein cholesterol is 43 mg/dL, low-density lipoprotein cholesterol is 121 mg/dL, and triglycerides level is 238 mg/dL. Electrocardiography reveals a QTc interval of 426 ms. Mr. S’s blood pressure is 105/65 mm Hg. Based on this clinic visit, the treatment team decides to change Mr. S’s antipsychotic.
Psychiatric illness and HIV/AIDS
There is a strong link between mental illness and HIV/AIDS; 50% or more of patients with HIV/AIDS have a comorbid psychiatric disorder.1 The prevalence of mental illness in patients with HIV/AIDS is reported to be 8 times higher than in those without HIV/AIDS.2 Depression, bipolar disorder, anxiety disorders, delirium, substance abuse, and schizophrenia have all been identified in persons receiving highly active antiretroviral therapy (HAART). Patients with HIV/AIDS and psychiatric illness have a decreased quality of life, poor adherence to medications, faster disease progression, and increased mortality. Care of these individuals is complicated by the stigma of HIV/AIDS and the prevalence of the illness in underserved populations, as well as the need for complex medication regimens and the possibility of drug–drug interactions (DDIs).1,2 If left untreated, psychiatric illness in patients with HIV/AIDS may lead to further transmission of HIV as a result of patients engaging in high-risk behaviors, along with poor adherence to HAART.3
Individuals diagnosed with schizophrenia, schizoaffective disorder, and bipolar disorder are at greater risk for HIV infection.3 Patients with HIV/AIDS with primary psychosis may have poor medication adherence rates due to illness-related confusion or paranoia about medications. Furthermore, they may lack the resources to manage the complications and stress related to living with HIV/AIDS.
New-onset, or secondary psychosis, has been reported in individuals with late-stage HIV/AIDS with CD4 counts <200 who have not been diagnosed with a psychotic disorder previously.3 These patients may experience more persecutory and grandiose delusions rather than hallucinations. Neuropsychiatric symptoms in patients with HIV/AIDS may be due to the presence of HIV or other infections in the CNS, tumors, or other inflammatory illnesses. Medications that have been implicated in neuropsychiatric symptoms include efavirenz, rilpivirine, and other HAART regimens; interferon; metoclopramide; corticosteroids; muscle relaxants; and clonidine. It is possible that symptoms may continue even after the medications are discontinued.3
Antipsychotics remain the treatment of choice for psychosis in HIV/AIDS, regardless of the cause of the symptoms. Many factors must be taken into consideration when choosing an antipsychotic, such as DDIs, adverse effect profiles, patient history of antipsychotic use, cost, and patient preference. Here we focus primarily on DDIs and adverse effect profiles.
When treating psychosis in patients with HIV/AIDS, it is crucial to consider potential DDIs. Many antipsychotics and antiretroviral medications utilize cytochrome P450 (CYP) enzymes for their metabolism. The CYP enzyme system is responsible for the oxidative reactions that constitute the phase I reactions necessary for the metabolism of most drugs. Inhibition and induction of CYP enzymes are among the most common causes of pharmacokinetic DDIs. Antipsychotics are predominately metabolized by CYP3A4, CYP1A2, and CYP2D6.4
Continue to: The DDIs arise because...
The DDIs arise because many antiretroviral medications inhibit, or in some cases, induce, these CYP enzymes, thereby altering substrate-drug metabolism. Inhibiting a CYP enzyme pathway can decrease substrate-drug clearance and lead to increased levels of that drug. This, in turn, can cause an increased risk of adverse effects, such as extrapyramidal symptoms (EPS) or QTc prolongation, which are both types of pharmacodynamic DDIs.4-28 However, because antipsychotics often have more than one pathway of metabolism, it can be challenging to understand the full effect of CYP-related DDIs. Furthermore, CYP enzyme inducers can decrease drug levels, and in the case of antipsychotics, lead to subtherapeutic responses.
Table 1,6-14,19-28 Table 2,15-28 Table 3,6-14,19-28 and Table 415-28 list many of the known CYP enzyme-related DDIs that may occur with combination antipsychotic and antiretroviral medication therapy and aim to predict CYP induction or inhibition based on a particular combination. The following antiretroviral medications do not have any CYP-related interactions and therefore are not included in the Tables: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir disoproxil, zidovudine, enfuvirtide, maraviroc, and raltegravir.
These Tables include the risk ratings for all D-rated (consider alternative therapy) and X-rated (avoid therapy) combinations. The majority of D-rated interactions are caused by CYP inhibition or induction that could potentially lead to altered antipsychotic levels. The majority of X-rated interactions are caused by increased QTc prolongation that may or may not be due to CYP-related DDIs. For example, paliperidone is not believed to be affected by the CYP enzyme system, but it does present a high risk of QTc prolongation on its own. When combined with an antiretroviral that also has a high risk of QTc prolongation, such as lopinavir, then the risk further increases.
Non-nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) are the antiretroviral medications most likely to cause DDIs with antipsychotics. Other antiretroviral classes, such as nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), fusion inhibitors, chemokine receptor 5 inhibitors, and integrase inhibitors, are not associated with CYP-related DDIs.19-28 For the most part, the severity of the CYP-related DDIs have not been well studied; therefore, most recommendations call for closer patient monitoring when combining antiretroviral medications and antipsychotics.6-18 The goal is to monitor for any changes in medication efficacy or adverse effects.
Continue to: Consider adverse effect profiles
Consider adverse effect profiles
When selecting an antipsychotic agent for a patient receiving HIV therapy, also consider adverse effect profiles. The emergence of adverse effects can greatly impact patients’ quality of life, leading to consequences of medication nonadherence and exacerbation of mental illness.
Extrapyramidal symptoms. Patients with HIV have a higher sensitivity to treatment-emergent EPS from antipsychotics.2 This sensitivity is generally thought to arise from the involvement of HIV on the basal ganglia. Historically, psychotic symptoms in HIV have been managed with second-generation antipsychotics (SGAs) at the lowest effective dose because these medications are less likely to cause EPS.1,29 The antipsychotic with the lowest rate of EPS is clozapine, followed by quetiapine, olanzapine, ziprasidone, and aripiprazole. Conversely, high-potency first-generation antipsychotics (FGAs) have the highest rates of EPS, followed by intermediate-potency FGAs and risperidone.30
Metabolic disturbances are another concern with concomitant antipsychotic/antiretroviral therapy. Patients with HIV who are receiving NRTIs or PIs can present with drug-induced lipodystrophy syndrome, which is associated with hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, and ultimately may cause metabolic syndrome.29 The prevalence of metabolic syndrome in patients receiving PI therapy has a vast range—2% to 84%—which can be attributed to inconsistent definitions, criteria, and assessment methodology.29 Use of a PI is considered to be the most prominent risk factor for developing lipodystrophy.29 Among the PIs, metabolic disturbances in regards to lipids are most often seen with lopinavir/ritonavir (LPV/r), saquinavir/ritonavir, tipranavir/ritonavir, and fosamprenavir/ritonavir.31 In comparison with LPV/r, darunavir showed improvement in lipids.32 Atazanavir (ATV) boosted with ritonavir has not shown clinically significant adverse effects on lipids.31 Additionally, amprenavir, LPV/r, and ritonavir demonstrated more glucose uptake inhibition via blockade of the glucose transporter type 4 than ATV.31 Of the NRTIs, lipodystrophy syndrome is most commonly seen with stavudine, which is used minimally in practice.2
The rates of metabolic disturbance with antipsychotic use range from 2% to 36%.2 The American Psychiatric Association recommends selecting one of the SGAs least likely to affect metabolic parameters.29 Aripiprazole and ziprasidone are associated with the lowest risk of weight gain, hyperglycemia, and hyperlipidemia. They are followed by risperidone and quetiapine, which are associated with moderate risk, and then clozapine and olanzapine, which are associated with high risk.2,30,33
Continue to: Management of metabolic adverse effects involves...
Management of metabolic adverse effects involves switching the antiretroviral agent and/or antipsychotic agent to an alternative associated with lower metabolic risk. Antipsychotics with low metabolic risk include aripiprazole, lurasidone, and ziprasidone. Lifestyle modifications are encouraged. Additionally, medication interventions, such as metformin, are also recommended in patients meeting criteria for pre-diabetes or type 2 diabetes mellitus.2 Lipid panels and metabolic parameters should be monitored periodically, according to guidelines.25,34
Bone marrow toxicity and blood dyscrasias. Lastly, consider the risk of bone marrow suppression. Patients receiving clozapine for treatment-resistant schizophrenia should be closely monitored for neutropenia and agranulocytosis. Although zidovudine is rarely used, its use is associated with adverse myelosuppressive effects, and the combination of clozapine and zidovudine could pose danger to the patient.2,35,36
CASE CONTINUED
Because Mr. S’s diagnosis of HIV puts him at a higher risk of developing EPS, and because he is already experiencing increased wrist rigidity, the treatment team decides to switch his antipsychotic therapy to an agent with a lower risk of EPS. His comorbidities, including type 2 diabetes mellitus, hypertension, and hyperlipidemia, are taken into account, and an SGA with a benign metabolic profile is considered. Aripiprazole and ziprasidone are favorable options. However, because efavirenz, ATZ, and ritonavir may cause QTc prolongation, ziprasidone, the SGA with the highest rate of QTc prolongation, is not the preferred option.
Mr. S’s SGA therapy is switched from risperidone to aripiprazole. Because potential CYP-related interactions between aripiprazole and Mr. S’s current antiretroviral therapy could lead to increased aripiprazole levels. Mr. S is started on a low dose (5 mg/d) with the goal to titrate based on response and tolerability. Increased levels of aripiprazole may increase the risk of akathisia, drowsiness, headaches, and fatigue. Mr. S is monitored closely for improvement of EPS, adverse effects of medication, and metabolic parameters. Furthermore, if the treatment team believes there is a more preferred antipsychotic for the patient that it did not prescribe because of the risk of DDIs, it may be worthwhile to consider discussing the HAART regimen with the patient’s infectious disease treatment team.
Continue to: Acknowledgements
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Chillicothe Veterans Affairs Medical Center in Chillicothe, Ohio. The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Related Resources
- Cohen MA. HIV: How to provide compassionate care. Current Psychiatry. 2013;12(6):19-23,A,B.
- Khan AY, Zaidi SN. Reducing morbidity and mortality from common medical conditions in schizophrenia. Current Psychiatry. 2016;15(3):30-32,34-38,40.
Drug Brand Names
Abacavir • Ziagen
Amlodipine • Norvasc
Amprenavir • Agenerase
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion ER • Wellbutrin SR
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Darunavir • Prezista
Delavirdine • Rescriptor
Didanosine • Videx EC
Efavirenz • Sustiva
Efavirenz/emtricitabine/tenofovir disoproxil fumarate • Atripla
Enfuvirtide • Fuzeon
Emtricitabine • Emtriva
Etravirine • Intelence
Fluphenazine • Prolixin
Fosamprenavir • Lexiva
Gabapentin • Neurontin
Glipizide • Glucotrol
Haloperidol • Haldol
Iloperidone • Fanapt
Indinavir • Crixivan
Lamivudine • Epivir
Lopinavir/ritonavir • Kaletra
Loxapine • Loxitane
Lurasidone • Latuda
Maraviroc • Selzentry
Metformin • Glucophage
Metoclopramide • Reglan
Molindone • Moban
Nelfinavir • Viracept
Nevirapine • Viramune
Olanzapine • Zyprexa
Paliperidone • Invega
Perphenazine • Trilafon
Pimozide • Orap
Pravastatin • Pravachol
Quetiapine • Seroquel
Raltegravir • Isentress
Rilpivirine • Edurant
Risperidone • Risperdal
Ritonavir • Norvir
Saquinavir • Invirase
Stavudine • Zerit
Tenofovir disoproxil • Viread
Thioridazine • Mellaril
Thiothixene • Navane
Tipranavir • Aptivus
Trifluoperazine • Stelazine
Zidovudine • Retrovir
Ziprasidone • Geodon
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.
1. Freudenreich O, Goforth HW, Cozza KL, et al. Psychiatric treatment of persons with HIV/AIDS: An HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480-488.
2. Hill L, Lee KC. Pharmacotherapy considerations in patients with HIV and psychiatric disorders: Focus on antidepressants and antipsychotics. Ann Pharmacother. 2013;47(1):75-89.
3. Watkins CC, Treisman GJ. Neuropsychiatric complications of aging with HIV. J Neurovirol. 2012;18(4):277-290.
4. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99-112.
5. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. 2010;5(1):44-53
6. Reyataz [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
7. Prezista [package insert]. Toronto, ON: Janssen Inc.; 2017.
8. Lexiva [package insert]. Research Triangle Park, NC: Viiv Healthcare; 2017
9. Crixivan [package insert]. Whitehouse Station, NJ; Merck; 2016.
10. Kaletra [package insert]. North Chicago, IL: AbbVie Inc; 2017
11. Viracept [package insert]. Kirkland, QC: Pfizer Canada Inc.; 201
12. Norvir tablets and oral solution [package insert]. North Chicago, IL: AbbVie Inc; 2017
13. Invirase [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2016.
14. Aptivus [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2016.
15. Sustiva [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017
16. Intelence [package insert]. Titusville, NJ: Tibotec Pharmaceuticals; 2014.
17. Viramune [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2017.
18. Rescriptor [package insert]. Laval, QC: ViiV Healthcare ULC; 2013.
19. Ziagen [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
20. Videx EC [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2015.
21. Emtriva [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
22. Epivir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2017.
23. Zerit [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017.
24. Viread [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
25. Retrovir [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2015.
26. Fuzeon [package insert]. South San Francisco, CA: Genentech USA, Inc; 2017.
27. Selzentry [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2016.
28. Isentress [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017.
29. American Psychiatry Association. Practice guidelines for treatment of patients with HIV/AIDS. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/hivaids.pdf. Published 2010. Accessed March 1, 2018.
30. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 Schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
31. Hughes PJ, Cretton-Scott E, Teague A, et al. Protease inhibitors for patients with HIV-1 infection. P T. 2011;36(6):332-336,341-345.
32. Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389-1397.
33. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962.
34. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013;12(9):51-54.
35. Singh D, Goodkin K. Choice of antipsychotic in HIV-infected patients. J Clin Psychiatry. 2007;68(3):479-480.
36. Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96-S116.