User login
Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin (FULL)
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with a 5-fold increase in the risk of ischemic stroke and the risk increases with age.1-3 Oral anticoagulation (OAC) therapy effectively reduces the risk of ischemic stroke in patients with nonvalvular AF. However, OAC therapy carries a bleeding risk.4
Several bleeding risk scores have been developed and validated for patients with AF who are taking warfarin: HEMORR2HAGES (Hepatic or renal disease, Ethanol abuse, Malignancy, Older age, Reduced platelet count or function, Re-bleeding, Hypertension, Anemia, Genetic factors, Excessive fall risk, Stroke), ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation), and HAS-BLED (Hypertension, Abnormal renal and/or liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio [INR], Elderly, and Drugs and/or alcohol excess concomitantly).4,5 All 3 bleeding risk scores demonstrate only modest ability to predict clinically relevant bleeding in patients taking warfarin. The HAS-BLED score was superior to HEMORR2HAGES and ATRIA for predicting any clinically relevant bleeding and was the only bleeding risk score that demonstrated significant predictive performance for intracranial hemorrhage.5 Compared with HEMORR2HAGES, the HAS-BLED score is simpler to use and to assess risk factors that can be gathered from medical history or routinely tested in patients with AF.4 Unlike HAS-BLED, HEMORR2HAGES and ATRIA do not consider medications that could increase the risk of bleeding.
Despite the availability of validated bleeding risk scores, clinical application of these measures should not be used to exclude a patient from OAC therapy for patients who reach a threshold score. Rather, current guideline and expert consensus agree with the recommendation to use bleeding risk scores to identify risk factors and address those factors that are modifiable to reduce the risk of anticoagulant-associated major bleeding.6-8
The authors identified modifiable bleeding risk factors using the HAS-BLED score and evaluated pharmacist interventions to correct these factors in patients with nonvalvular AF who are taking warfarin. To the authors’ knowledge, there have been no published studies evaluating interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED score.
Methods
Clinical pharmacy specialists (CPSs) in the primary care (PC) clinics at the Clement J. Zablocki VAMC (CJZVAMC) in Milwaukee, Wisconsin, have prescriptive authority within their scope of practice to manage smoking cessation and diseases, including anticoagulation, diabetes mellitus, heart failure, hypertension, and dyslipidemia. Patients who are on OAC therapy, including warfarin, receive comprehensive anticoagulation management from PC CPSs, including prescribing OAC therapy, education, dosage adjustments, and laboratory monitoring.
Patients were included in the HAS-BLED risk scoring and intervention if their warfarin therapy was managed by a PC CPS, had an active warfarin prescription with a diagnosis of nonvalvular AF in their problem list, and had ≥ 1 modifiable risk factor(s) from the HAS-BLED risk score. Modifiable risk factors evaluated were systolic blood pressure (SBP) > 160 mm Hg, an active prescription for VA or non-VA (which generally indicates over-the-counter [OTC] medication use) aspirin, clopidogrel, or a nonsteroidal anti-inflammatory drug (NSAID). Excess alcohol consumption was not listed as a modifiable risk factor in this assessment because nearly all the anticoagulation patients already receive regular recommendations to minimize alcohol use from the PC CPSs.
Patients were excluded from analysis if they had an indication for warfarin use other than nonvalvular AF, such as atrial flutter, acute/chronic deep vein thrombosis or pulmonary embolism, history of venous thromboembolism, peripheral vascular disease, or aortic or mitral mechanical valve. Patients also were excluded if they were on antiplatelet therapy for unstable coronary artery disease (CAD), experienced acute coronary syndrome within the past 1 year, history of stent placement, carotid endarterectomy, carotid stenosis, or noncardioembolic stroke and no other modifiable risk factors. Last, patients were excluded if clinic SBP readings were > 160 mm Hg but there was documented white coat hypertension or home SBP readings < 160 mm Hg.
The following definitions or measurements were used for assessing the HAS-BLED bleeding risk score4:
- Uncontrolled hypertension: most recently charted SBP > 160 mm Hg;
- Abnormal renal function: dialysis, renal transplant, serum creatinine > 2.26 mg/dL;
- Abnormal liver function: chronic hepatic disease, biochemical evidence of significant hepatic derangement (bilirubin > 2 × upper limit of normal and/or AST/ALT/alkaline phosphatase > 3 × upper limit of normal);
- Stroke: including history of transient ischemic attack;
- Bleeding history or predisposition: history of major bleeding (intracranial and/or any bleeding requiring hospitalization and/or causing a decrease in hemoglobin (Hgb) level of > 2 g/dL and/or requiring blood transfusion), anemia (males: Hgb < 13 g/dL; females: Hgb < 12 g/dL);
- Labile INR: percentage of INRs in therapeutic range < 60% (using the CJZVAMC anticoagulation management tool, which calculates percentage of INRs in goal reported since the first visit);
- Geriatric: age > 65 years at initial assessment;
- Concomitant drug use (VA prescription or non-VA medication list): aspirin, clopidogrel, or NSAID; and
- Alcohol in excess: > 8 alcohol servings per week from chart documentation of the patient’s self-report.
In the HAS-BLED bleeding risk score, patients receive 1 point for each component for a maximum score of 9 points. The score is stratified into low (0 points), intermediate (1 to 2 points), and high (≥ 3 points) bleeding risk.4
The HAS-BLED risk factors were obtained from patient chart review, including problem list, laboratory results, and PC CPS anticoagulation notes. Interventions included primary care provider (PCP) notification of elevated BP and offer of BP management by a PC CPS, patient education and/or PCP contact to discontinue concurrent NSAID or addition of a proton pump inhibitor (PPI) based on bleeding risk factor if the NSAID was deemed necessary, and discontinuation of concomitant antiplatelet drug(s) or reduction in aspirin dosage in consultation with patient’s PCP and cardiologist.9 In order to complete the initial HAS-BLED assessment and implement interventions, a note template was developed and entered into the electronic health record (EHR) that identified the patient’s modifiable risk factors.
Once the PCP and cardiologist (if applicable) responded to the note, by either accepting or declining the PC CPS recommendation(s), the HAS-BLED score was recalculated and recorded. If the provider did not respond to the initial note, an attempt was made to follow up at 3 months and at 6 months if necessary. If the provider did not respond at 6 months, the nonresponse was documented. For patients whose PCP requested PC CPS management of BP, the HAS-BLED score was recalculated 6 months after response from the PCP.
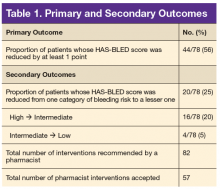
The primary outcome was the proportion of patients whose HAS-BLED score was reduced by at least 1 point. Secondary outcomes included the proportion of patients whose HAS-BLED score was reduced from one category of bleeding risk to a lesser one, total number of pharmacist interventions completed, number of pharmacist interventions made of each type (BP management, NSAID use, or antiplatelet drug use), and PCP acceptance rate.
Results
A total of 897 patients taking warfarin received anticoagulation management by a PC CPS at CJZVAMC in 2015. Of these, 819 patients were excluded based on the exclusion criteria (eFigure).
Seventy-eight patients were included in the assessment. Baseline HAS-BLED scores were calculated, and recommendations were made via an EHR progress note to the PCP and cardiologist (if applicable). Recommended interventions in the 78 patients resulted in 44 patients (56%) who experienced reduction in their HAS-BLED score by at least 1 point (Table 1). Twenty patients (25%) saw their HAS-BLED category reduced from a higher level of bleeding risk to a lower risk. The average HAS-BLED score in the 44 patients was 2.38 before intervention and 1.55 after the intervention.
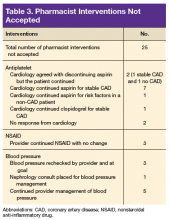
In 10 patients, the HAS-BLED score did not decrease despite accepted PC CPS intervention. Specifically, 7 patients were on both an antiplatelet agent and NSAID. As a result of the pharmacist intervention, the NSAID was discontinued, but the antiplatelet remained because of stent placement or carotid stenosis. In 1 patient, the aspirin dosage was decreased from 325 to 81 mg/d. In 2 patients where NSAID use was deemed necessary—meloxicam in both cases—a PPI was ordered based on bleeding risk.9
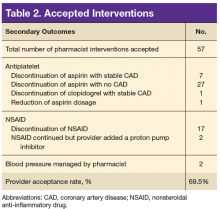
A total of 82 interventions were recommended; 57 interventions were accepted, resulting in a provider acceptance rate of 69.5% (Tables 2 and 3). Thirty-five of the accepted interventions (61%) involved discontinuing an antiplatelet (aspirin or clopidogrel) in consultation with the patient’s PCP and cardiologist. Twenty-seven of these patients had no documented CAD, and 8 of the patients had stable CAD. Seventeen (30%) of the accepted recommendations were for discontinuing NSAID therapy, 2 (4%) were for BP management by a PC CPS, 2 (4%) for addition of PPI with continued NSAID use (meloxicam), and 1 (1%) for decreasing aspirin dosage from 325 to 81 mg/d. The NSAIDs that were discontinued included ibuprofen, indomethacin, meloxicam, and naproxen.
Discussion
This project is the first, to the authors’ knowledge, to evaluate pharmacist interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED bleeding risk score. Most of the patients with nonvalvular AF in the PC clinics did not have modifiable bleeding risk factors. However, of the patients who received a recommendation to reduce a specific modifiable risk factor, most of the interventions were accepted by PCPs and
Most of the interventions recommended evaluating the use of antiplatelet agents, particularly aspirin. The benefits of antiplatelet therapy for secondary prevention of cardiovascular disease are well established. However, for AF patients on OAC therapy, the concomitant use of antiplatelet therapy significantly increases the risk of bleeding and should be reserved for high-risk patients.10 The 2012 American College of Chest Physicians guidelines support the use of OAC monotherapy in patients with AF with stable CAD, including patients with a myocardial infarction or percutaneous coronary intervention more than 1 year previously, which has been corroborated with guideline and expert consensus recommendations released in 2016.7,8,10,11
For patients taking warfarin for AF without CAD, the possible benefit of concomitant aspirin therapy for primary prevention is outweighed by the increased risk of major bleeding.12 Furthermore, warfarin monotherapy has been shown to be effective in primary prevention of CAD and seems to have cardiovascular benefit for secondary prevention but with increased bleeding.13,14 As a result, through the exclusion criteria this project aimed to evaluate warfarin patients with AF at low risk of cardiovascular events who might be taking unnecessary concurrent antiplatelet therapy.
More than one-half of the interventions involved discontinuing antiplatelet therapy. Chart reviews revealed a lack of documentation for the indication and intended duration of antiplatelet therapy. In many patients, it is likely that aspirin use predated AF diagnosis and warfarin initiation. In some of these cases, it would have been appropriate to discontinue aspirin when starting warfarin use. Although there is guidance to support the use of OAC monotherapy in patients with AF with stable CAD, the patient’s provider and cardiologist made the decision to discontinue an antiplatelet agent after weighing benefits and risks. Regardless of the outcome, this analysis revealed the importance and need for routine review of antiplatelet therapy and documenting the rationale for antiplatelet use in addition to anticoagulation.
The second largest category of interventions accepted was for evaluation of NSAID use. A 2014 study by Lamberts and colleagues found that concomitant use of oral anticoagulants and NSAIDs conferred an independent risk for major bleeding and thromboembolism in patients with AF.15 The increase in serious bleeding (absolute risk difference of 2.5 events per 1,000 patients) was observed even with short-term NSAID exposure of 14 days across all NSAID types (selective COX-2 inhibitors or nonselective NSAIDs). In addition, there was an incremental increase in bleeding risk with high NSAID dosages. The risk of serious bleeding was even greater when an NSAID is added to OAC therapy and aspirin. Seven out of 17 warfarin patients (41%) who were taking an NSAID also were on an antiplatelet agent. As a result of the pharmacist interventions, NSAIDs were discontinued in all of these patients, but the antiplatelet remained because of stent placement or carotid stenosis.
This analysis captured only those patients with a documented active prescription or self-reported OTC use of an NSAID. It is unknown how many patients might take OTC NSAIDs occasionally but not report this use to a provider or pharmacist. Primary care CPSs educate patients to not use NSAIDs while taking warfarin during their initial visit and periodically thereafter; however, with the number of different NSAIDs available without a prescription and the various brand and generic names offered, it can be difficult for patients to understand what they should or should not take for minor pain or fever. Therefore, it is imperative that NSAID use is reviewed regularly at anticoagulant follow-up visits and patients are educated about alternative OTC agents for pain relief (eg, acetaminophen, topical agents, heating pad) when necessary. It also is equally important for PCPs to weigh the benefit vs risk for each patient before prescribing an NSAID if alternatives have been exhausted especially if the patient also is taking an OAC and antiplatelet agent.
The smallest number of interventions completed was for BP management. According to the HAS-BLED bleeding risk score, BP management was recommended only if the most recent clinic SBP was > 160 mm Hg, excluding patients with documented white-coat syndrome or home SBP readings < 160 mm Hg. One potential explanation for the small number of patients with SBP > 160 mm Hg is that for many of the patients taking warfarin, the PC CPSs at CJZVAMC have been involved in their BP management through earlier consultation by providers.
Limitations
A limitation of the BP component of the HAS-BLED score was that the assessment of BP for this project was only one point in time. In 3 cases, the SBP was > 160 mm Hg only during the most recent measurement, and these patients had normal BP readings on return to the clinic for follow-up. This category of recommendation also took more time for follow-up because a PC CPS would need to evaluate the patient in clinic, implement changes to BP medications, and follow-up at subsequent visits. Although some PCPs felt that the patient did not need pharmacist intervention, the elevated SBPs were brought to the provider’s attention, and some patients received further monitoring by the PCP or through a specialty clinic (eg, nephrology).
Another limitation of this project was that the bleeding risk evaluation occurred at only 1 visit. Patients’ medications and medical issues often change with time. Therefore, it is important to implement a process to regularly review (eg, annually) patients’ bleeding risk factors and to identify and act on modifiable risk factors. Another limitation was a lack of a comparator group and the time frame of the evaluation. As a result, the authors were unable to evaluate bleeding outcomes because of the small sample size and limited time frame. Future studies could consider evaluating bleeding events as an outcome, including additional modifiable risk factors, such as excess alcohol and labile INR, expanding the review to patients taking warfarin for indications other than AF, and review of patients on direct-acting oral anticoagulants (DOACs) with AF; keeping in mind that currently available bleeding risk calculators were developed for patients taking warfarin, not DOACs with AF. Patients could be counselled on reducing alcohol intake or switching to a DOAC if INR is labile despite adherence.
Conclusion
This quality improvement project successfully implemented use of the HAS-BLED bleeding risk score to identify and reduce modifiable bleeding risk factors in patients with AF taking warfarin. Pharmacist intervention resulted in a reduction of HAS-BLED scores and bleeding risk categories.
Click here to read the digital edition.
1. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042-1046.
2. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371-2379.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
4. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
5. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60(9):861-867.
6. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
7. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
8. Ruff CT, Ansell JE, Becker RC, et al. North American thrombosis forum, AF action initiative consensus document. Am J Med. 2016;129(suppl 5):S1-S29.
9. Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728-738.
10. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation. Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
11. Macle L, Cairns J, Leblanc K, et al; CCS Atrial Fibrillation Guidelines Committee. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170-1185.
12. Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med. 2007;167(2):117-124.
13. The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351(9098):233-241.
14. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
15. Lamberts M, Lip GY, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with a 5-fold increase in the risk of ischemic stroke and the risk increases with age.1-3 Oral anticoagulation (OAC) therapy effectively reduces the risk of ischemic stroke in patients with nonvalvular AF. However, OAC therapy carries a bleeding risk.4
Several bleeding risk scores have been developed and validated for patients with AF who are taking warfarin: HEMORR2HAGES (Hepatic or renal disease, Ethanol abuse, Malignancy, Older age, Reduced platelet count or function, Re-bleeding, Hypertension, Anemia, Genetic factors, Excessive fall risk, Stroke), ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation), and HAS-BLED (Hypertension, Abnormal renal and/or liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio [INR], Elderly, and Drugs and/or alcohol excess concomitantly).4,5 All 3 bleeding risk scores demonstrate only modest ability to predict clinically relevant bleeding in patients taking warfarin. The HAS-BLED score was superior to HEMORR2HAGES and ATRIA for predicting any clinically relevant bleeding and was the only bleeding risk score that demonstrated significant predictive performance for intracranial hemorrhage.5 Compared with HEMORR2HAGES, the HAS-BLED score is simpler to use and to assess risk factors that can be gathered from medical history or routinely tested in patients with AF.4 Unlike HAS-BLED, HEMORR2HAGES and ATRIA do not consider medications that could increase the risk of bleeding.
Despite the availability of validated bleeding risk scores, clinical application of these measures should not be used to exclude a patient from OAC therapy for patients who reach a threshold score. Rather, current guideline and expert consensus agree with the recommendation to use bleeding risk scores to identify risk factors and address those factors that are modifiable to reduce the risk of anticoagulant-associated major bleeding.6-8
The authors identified modifiable bleeding risk factors using the HAS-BLED score and evaluated pharmacist interventions to correct these factors in patients with nonvalvular AF who are taking warfarin. To the authors’ knowledge, there have been no published studies evaluating interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED score.
Methods
Clinical pharmacy specialists (CPSs) in the primary care (PC) clinics at the Clement J. Zablocki VAMC (CJZVAMC) in Milwaukee, Wisconsin, have prescriptive authority within their scope of practice to manage smoking cessation and diseases, including anticoagulation, diabetes mellitus, heart failure, hypertension, and dyslipidemia. Patients who are on OAC therapy, including warfarin, receive comprehensive anticoagulation management from PC CPSs, including prescribing OAC therapy, education, dosage adjustments, and laboratory monitoring.
Patients were included in the HAS-BLED risk scoring and intervention if their warfarin therapy was managed by a PC CPS, had an active warfarin prescription with a diagnosis of nonvalvular AF in their problem list, and had ≥ 1 modifiable risk factor(s) from the HAS-BLED risk score. Modifiable risk factors evaluated were systolic blood pressure (SBP) > 160 mm Hg, an active prescription for VA or non-VA (which generally indicates over-the-counter [OTC] medication use) aspirin, clopidogrel, or a nonsteroidal anti-inflammatory drug (NSAID). Excess alcohol consumption was not listed as a modifiable risk factor in this assessment because nearly all the anticoagulation patients already receive regular recommendations to minimize alcohol use from the PC CPSs.
Patients were excluded from analysis if they had an indication for warfarin use other than nonvalvular AF, such as atrial flutter, acute/chronic deep vein thrombosis or pulmonary embolism, history of venous thromboembolism, peripheral vascular disease, or aortic or mitral mechanical valve. Patients also were excluded if they were on antiplatelet therapy for unstable coronary artery disease (CAD), experienced acute coronary syndrome within the past 1 year, history of stent placement, carotid endarterectomy, carotid stenosis, or noncardioembolic stroke and no other modifiable risk factors. Last, patients were excluded if clinic SBP readings were > 160 mm Hg but there was documented white coat hypertension or home SBP readings < 160 mm Hg.
The following definitions or measurements were used for assessing the HAS-BLED bleeding risk score4:
- Uncontrolled hypertension: most recently charted SBP > 160 mm Hg;
- Abnormal renal function: dialysis, renal transplant, serum creatinine > 2.26 mg/dL;
- Abnormal liver function: chronic hepatic disease, biochemical evidence of significant hepatic derangement (bilirubin > 2 × upper limit of normal and/or AST/ALT/alkaline phosphatase > 3 × upper limit of normal);
- Stroke: including history of transient ischemic attack;
- Bleeding history or predisposition: history of major bleeding (intracranial and/or any bleeding requiring hospitalization and/or causing a decrease in hemoglobin (Hgb) level of > 2 g/dL and/or requiring blood transfusion), anemia (males: Hgb < 13 g/dL; females: Hgb < 12 g/dL);
- Labile INR: percentage of INRs in therapeutic range < 60% (using the CJZVAMC anticoagulation management tool, which calculates percentage of INRs in goal reported since the first visit);
- Geriatric: age > 65 years at initial assessment;
- Concomitant drug use (VA prescription or non-VA medication list): aspirin, clopidogrel, or NSAID; and
- Alcohol in excess: > 8 alcohol servings per week from chart documentation of the patient’s self-report.
In the HAS-BLED bleeding risk score, patients receive 1 point for each component for a maximum score of 9 points. The score is stratified into low (0 points), intermediate (1 to 2 points), and high (≥ 3 points) bleeding risk.4
The HAS-BLED risk factors were obtained from patient chart review, including problem list, laboratory results, and PC CPS anticoagulation notes. Interventions included primary care provider (PCP) notification of elevated BP and offer of BP management by a PC CPS, patient education and/or PCP contact to discontinue concurrent NSAID or addition of a proton pump inhibitor (PPI) based on bleeding risk factor if the NSAID was deemed necessary, and discontinuation of concomitant antiplatelet drug(s) or reduction in aspirin dosage in consultation with patient’s PCP and cardiologist.9 In order to complete the initial HAS-BLED assessment and implement interventions, a note template was developed and entered into the electronic health record (EHR) that identified the patient’s modifiable risk factors.
Once the PCP and cardiologist (if applicable) responded to the note, by either accepting or declining the PC CPS recommendation(s), the HAS-BLED score was recalculated and recorded. If the provider did not respond to the initial note, an attempt was made to follow up at 3 months and at 6 months if necessary. If the provider did not respond at 6 months, the nonresponse was documented. For patients whose PCP requested PC CPS management of BP, the HAS-BLED score was recalculated 6 months after response from the PCP.
The primary outcome was the proportion of patients whose HAS-BLED score was reduced by at least 1 point. Secondary outcomes included the proportion of patients whose HAS-BLED score was reduced from one category of bleeding risk to a lesser one, total number of pharmacist interventions completed, number of pharmacist interventions made of each type (BP management, NSAID use, or antiplatelet drug use), and PCP acceptance rate.
Results
A total of 897 patients taking warfarin received anticoagulation management by a PC CPS at CJZVAMC in 2015. Of these, 819 patients were excluded based on the exclusion criteria (eFigure).
Seventy-eight patients were included in the assessment. Baseline HAS-BLED scores were calculated, and recommendations were made via an EHR progress note to the PCP and cardiologist (if applicable). Recommended interventions in the 78 patients resulted in 44 patients (56%) who experienced reduction in their HAS-BLED score by at least 1 point (Table 1). Twenty patients (25%) saw their HAS-BLED category reduced from a higher level of bleeding risk to a lower risk. The average HAS-BLED score in the 44 patients was 2.38 before intervention and 1.55 after the intervention.
In 10 patients, the HAS-BLED score did not decrease despite accepted PC CPS intervention. Specifically, 7 patients were on both an antiplatelet agent and NSAID. As a result of the pharmacist intervention, the NSAID was discontinued, but the antiplatelet remained because of stent placement or carotid stenosis. In 1 patient, the aspirin dosage was decreased from 325 to 81 mg/d. In 2 patients where NSAID use was deemed necessary—meloxicam in both cases—a PPI was ordered based on bleeding risk.9
A total of 82 interventions were recommended; 57 interventions were accepted, resulting in a provider acceptance rate of 69.5% (Tables 2 and 3). Thirty-five of the accepted interventions (61%) involved discontinuing an antiplatelet (aspirin or clopidogrel) in consultation with the patient’s PCP and cardiologist. Twenty-seven of these patients had no documented CAD, and 8 of the patients had stable CAD. Seventeen (30%) of the accepted recommendations were for discontinuing NSAID therapy, 2 (4%) were for BP management by a PC CPS, 2 (4%) for addition of PPI with continued NSAID use (meloxicam), and 1 (1%) for decreasing aspirin dosage from 325 to 81 mg/d. The NSAIDs that were discontinued included ibuprofen, indomethacin, meloxicam, and naproxen.
Discussion
This project is the first, to the authors’ knowledge, to evaluate pharmacist interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED bleeding risk score. Most of the patients with nonvalvular AF in the PC clinics did not have modifiable bleeding risk factors. However, of the patients who received a recommendation to reduce a specific modifiable risk factor, most of the interventions were accepted by PCPs and
Most of the interventions recommended evaluating the use of antiplatelet agents, particularly aspirin. The benefits of antiplatelet therapy for secondary prevention of cardiovascular disease are well established. However, for AF patients on OAC therapy, the concomitant use of antiplatelet therapy significantly increases the risk of bleeding and should be reserved for high-risk patients.10 The 2012 American College of Chest Physicians guidelines support the use of OAC monotherapy in patients with AF with stable CAD, including patients with a myocardial infarction or percutaneous coronary intervention more than 1 year previously, which has been corroborated with guideline and expert consensus recommendations released in 2016.7,8,10,11
For patients taking warfarin for AF without CAD, the possible benefit of concomitant aspirin therapy for primary prevention is outweighed by the increased risk of major bleeding.12 Furthermore, warfarin monotherapy has been shown to be effective in primary prevention of CAD and seems to have cardiovascular benefit for secondary prevention but with increased bleeding.13,14 As a result, through the exclusion criteria this project aimed to evaluate warfarin patients with AF at low risk of cardiovascular events who might be taking unnecessary concurrent antiplatelet therapy.
More than one-half of the interventions involved discontinuing antiplatelet therapy. Chart reviews revealed a lack of documentation for the indication and intended duration of antiplatelet therapy. In many patients, it is likely that aspirin use predated AF diagnosis and warfarin initiation. In some of these cases, it would have been appropriate to discontinue aspirin when starting warfarin use. Although there is guidance to support the use of OAC monotherapy in patients with AF with stable CAD, the patient’s provider and cardiologist made the decision to discontinue an antiplatelet agent after weighing benefits and risks. Regardless of the outcome, this analysis revealed the importance and need for routine review of antiplatelet therapy and documenting the rationale for antiplatelet use in addition to anticoagulation.
The second largest category of interventions accepted was for evaluation of NSAID use. A 2014 study by Lamberts and colleagues found that concomitant use of oral anticoagulants and NSAIDs conferred an independent risk for major bleeding and thromboembolism in patients with AF.15 The increase in serious bleeding (absolute risk difference of 2.5 events per 1,000 patients) was observed even with short-term NSAID exposure of 14 days across all NSAID types (selective COX-2 inhibitors or nonselective NSAIDs). In addition, there was an incremental increase in bleeding risk with high NSAID dosages. The risk of serious bleeding was even greater when an NSAID is added to OAC therapy and aspirin. Seven out of 17 warfarin patients (41%) who were taking an NSAID also were on an antiplatelet agent. As a result of the pharmacist interventions, NSAIDs were discontinued in all of these patients, but the antiplatelet remained because of stent placement or carotid stenosis.
This analysis captured only those patients with a documented active prescription or self-reported OTC use of an NSAID. It is unknown how many patients might take OTC NSAIDs occasionally but not report this use to a provider or pharmacist. Primary care CPSs educate patients to not use NSAIDs while taking warfarin during their initial visit and periodically thereafter; however, with the number of different NSAIDs available without a prescription and the various brand and generic names offered, it can be difficult for patients to understand what they should or should not take for minor pain or fever. Therefore, it is imperative that NSAID use is reviewed regularly at anticoagulant follow-up visits and patients are educated about alternative OTC agents for pain relief (eg, acetaminophen, topical agents, heating pad) when necessary. It also is equally important for PCPs to weigh the benefit vs risk for each patient before prescribing an NSAID if alternatives have been exhausted especially if the patient also is taking an OAC and antiplatelet agent.
The smallest number of interventions completed was for BP management. According to the HAS-BLED bleeding risk score, BP management was recommended only if the most recent clinic SBP was > 160 mm Hg, excluding patients with documented white-coat syndrome or home SBP readings < 160 mm Hg. One potential explanation for the small number of patients with SBP > 160 mm Hg is that for many of the patients taking warfarin, the PC CPSs at CJZVAMC have been involved in their BP management through earlier consultation by providers.
Limitations
A limitation of the BP component of the HAS-BLED score was that the assessment of BP for this project was only one point in time. In 3 cases, the SBP was > 160 mm Hg only during the most recent measurement, and these patients had normal BP readings on return to the clinic for follow-up. This category of recommendation also took more time for follow-up because a PC CPS would need to evaluate the patient in clinic, implement changes to BP medications, and follow-up at subsequent visits. Although some PCPs felt that the patient did not need pharmacist intervention, the elevated SBPs were brought to the provider’s attention, and some patients received further monitoring by the PCP or through a specialty clinic (eg, nephrology).
Another limitation of this project was that the bleeding risk evaluation occurred at only 1 visit. Patients’ medications and medical issues often change with time. Therefore, it is important to implement a process to regularly review (eg, annually) patients’ bleeding risk factors and to identify and act on modifiable risk factors. Another limitation was a lack of a comparator group and the time frame of the evaluation. As a result, the authors were unable to evaluate bleeding outcomes because of the small sample size and limited time frame. Future studies could consider evaluating bleeding events as an outcome, including additional modifiable risk factors, such as excess alcohol and labile INR, expanding the review to patients taking warfarin for indications other than AF, and review of patients on direct-acting oral anticoagulants (DOACs) with AF; keeping in mind that currently available bleeding risk calculators were developed for patients taking warfarin, not DOACs with AF. Patients could be counselled on reducing alcohol intake or switching to a DOAC if INR is labile despite adherence.
Conclusion
This quality improvement project successfully implemented use of the HAS-BLED bleeding risk score to identify and reduce modifiable bleeding risk factors in patients with AF taking warfarin. Pharmacist intervention resulted in a reduction of HAS-BLED scores and bleeding risk categories.
Click here to read the digital edition.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with a 5-fold increase in the risk of ischemic stroke and the risk increases with age.1-3 Oral anticoagulation (OAC) therapy effectively reduces the risk of ischemic stroke in patients with nonvalvular AF. However, OAC therapy carries a bleeding risk.4
Several bleeding risk scores have been developed and validated for patients with AF who are taking warfarin: HEMORR2HAGES (Hepatic or renal disease, Ethanol abuse, Malignancy, Older age, Reduced platelet count or function, Re-bleeding, Hypertension, Anemia, Genetic factors, Excessive fall risk, Stroke), ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation), and HAS-BLED (Hypertension, Abnormal renal and/or liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio [INR], Elderly, and Drugs and/or alcohol excess concomitantly).4,5 All 3 bleeding risk scores demonstrate only modest ability to predict clinically relevant bleeding in patients taking warfarin. The HAS-BLED score was superior to HEMORR2HAGES and ATRIA for predicting any clinically relevant bleeding and was the only bleeding risk score that demonstrated significant predictive performance for intracranial hemorrhage.5 Compared with HEMORR2HAGES, the HAS-BLED score is simpler to use and to assess risk factors that can be gathered from medical history or routinely tested in patients with AF.4 Unlike HAS-BLED, HEMORR2HAGES and ATRIA do not consider medications that could increase the risk of bleeding.
Despite the availability of validated bleeding risk scores, clinical application of these measures should not be used to exclude a patient from OAC therapy for patients who reach a threshold score. Rather, current guideline and expert consensus agree with the recommendation to use bleeding risk scores to identify risk factors and address those factors that are modifiable to reduce the risk of anticoagulant-associated major bleeding.6-8
The authors identified modifiable bleeding risk factors using the HAS-BLED score and evaluated pharmacist interventions to correct these factors in patients with nonvalvular AF who are taking warfarin. To the authors’ knowledge, there have been no published studies evaluating interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED score.
Methods
Clinical pharmacy specialists (CPSs) in the primary care (PC) clinics at the Clement J. Zablocki VAMC (CJZVAMC) in Milwaukee, Wisconsin, have prescriptive authority within their scope of practice to manage smoking cessation and diseases, including anticoagulation, diabetes mellitus, heart failure, hypertension, and dyslipidemia. Patients who are on OAC therapy, including warfarin, receive comprehensive anticoagulation management from PC CPSs, including prescribing OAC therapy, education, dosage adjustments, and laboratory monitoring.
Patients were included in the HAS-BLED risk scoring and intervention if their warfarin therapy was managed by a PC CPS, had an active warfarin prescription with a diagnosis of nonvalvular AF in their problem list, and had ≥ 1 modifiable risk factor(s) from the HAS-BLED risk score. Modifiable risk factors evaluated were systolic blood pressure (SBP) > 160 mm Hg, an active prescription for VA or non-VA (which generally indicates over-the-counter [OTC] medication use) aspirin, clopidogrel, or a nonsteroidal anti-inflammatory drug (NSAID). Excess alcohol consumption was not listed as a modifiable risk factor in this assessment because nearly all the anticoagulation patients already receive regular recommendations to minimize alcohol use from the PC CPSs.
Patients were excluded from analysis if they had an indication for warfarin use other than nonvalvular AF, such as atrial flutter, acute/chronic deep vein thrombosis or pulmonary embolism, history of venous thromboembolism, peripheral vascular disease, or aortic or mitral mechanical valve. Patients also were excluded if they were on antiplatelet therapy for unstable coronary artery disease (CAD), experienced acute coronary syndrome within the past 1 year, history of stent placement, carotid endarterectomy, carotid stenosis, or noncardioembolic stroke and no other modifiable risk factors. Last, patients were excluded if clinic SBP readings were > 160 mm Hg but there was documented white coat hypertension or home SBP readings < 160 mm Hg.
The following definitions or measurements were used for assessing the HAS-BLED bleeding risk score4:
- Uncontrolled hypertension: most recently charted SBP > 160 mm Hg;
- Abnormal renal function: dialysis, renal transplant, serum creatinine > 2.26 mg/dL;
- Abnormal liver function: chronic hepatic disease, biochemical evidence of significant hepatic derangement (bilirubin > 2 × upper limit of normal and/or AST/ALT/alkaline phosphatase > 3 × upper limit of normal);
- Stroke: including history of transient ischemic attack;
- Bleeding history or predisposition: history of major bleeding (intracranial and/or any bleeding requiring hospitalization and/or causing a decrease in hemoglobin (Hgb) level of > 2 g/dL and/or requiring blood transfusion), anemia (males: Hgb < 13 g/dL; females: Hgb < 12 g/dL);
- Labile INR: percentage of INRs in therapeutic range < 60% (using the CJZVAMC anticoagulation management tool, which calculates percentage of INRs in goal reported since the first visit);
- Geriatric: age > 65 years at initial assessment;
- Concomitant drug use (VA prescription or non-VA medication list): aspirin, clopidogrel, or NSAID; and
- Alcohol in excess: > 8 alcohol servings per week from chart documentation of the patient’s self-report.
In the HAS-BLED bleeding risk score, patients receive 1 point for each component for a maximum score of 9 points. The score is stratified into low (0 points), intermediate (1 to 2 points), and high (≥ 3 points) bleeding risk.4
The HAS-BLED risk factors were obtained from patient chart review, including problem list, laboratory results, and PC CPS anticoagulation notes. Interventions included primary care provider (PCP) notification of elevated BP and offer of BP management by a PC CPS, patient education and/or PCP contact to discontinue concurrent NSAID or addition of a proton pump inhibitor (PPI) based on bleeding risk factor if the NSAID was deemed necessary, and discontinuation of concomitant antiplatelet drug(s) or reduction in aspirin dosage in consultation with patient’s PCP and cardiologist.9 In order to complete the initial HAS-BLED assessment and implement interventions, a note template was developed and entered into the electronic health record (EHR) that identified the patient’s modifiable risk factors.
Once the PCP and cardiologist (if applicable) responded to the note, by either accepting or declining the PC CPS recommendation(s), the HAS-BLED score was recalculated and recorded. If the provider did not respond to the initial note, an attempt was made to follow up at 3 months and at 6 months if necessary. If the provider did not respond at 6 months, the nonresponse was documented. For patients whose PCP requested PC CPS management of BP, the HAS-BLED score was recalculated 6 months after response from the PCP.
The primary outcome was the proportion of patients whose HAS-BLED score was reduced by at least 1 point. Secondary outcomes included the proportion of patients whose HAS-BLED score was reduced from one category of bleeding risk to a lesser one, total number of pharmacist interventions completed, number of pharmacist interventions made of each type (BP management, NSAID use, or antiplatelet drug use), and PCP acceptance rate.
Results
A total of 897 patients taking warfarin received anticoagulation management by a PC CPS at CJZVAMC in 2015. Of these, 819 patients were excluded based on the exclusion criteria (eFigure).
Seventy-eight patients were included in the assessment. Baseline HAS-BLED scores were calculated, and recommendations were made via an EHR progress note to the PCP and cardiologist (if applicable). Recommended interventions in the 78 patients resulted in 44 patients (56%) who experienced reduction in their HAS-BLED score by at least 1 point (Table 1). Twenty patients (25%) saw their HAS-BLED category reduced from a higher level of bleeding risk to a lower risk. The average HAS-BLED score in the 44 patients was 2.38 before intervention and 1.55 after the intervention.
In 10 patients, the HAS-BLED score did not decrease despite accepted PC CPS intervention. Specifically, 7 patients were on both an antiplatelet agent and NSAID. As a result of the pharmacist intervention, the NSAID was discontinued, but the antiplatelet remained because of stent placement or carotid stenosis. In 1 patient, the aspirin dosage was decreased from 325 to 81 mg/d. In 2 patients where NSAID use was deemed necessary—meloxicam in both cases—a PPI was ordered based on bleeding risk.9
A total of 82 interventions were recommended; 57 interventions were accepted, resulting in a provider acceptance rate of 69.5% (Tables 2 and 3). Thirty-five of the accepted interventions (61%) involved discontinuing an antiplatelet (aspirin or clopidogrel) in consultation with the patient’s PCP and cardiologist. Twenty-seven of these patients had no documented CAD, and 8 of the patients had stable CAD. Seventeen (30%) of the accepted recommendations were for discontinuing NSAID therapy, 2 (4%) were for BP management by a PC CPS, 2 (4%) for addition of PPI with continued NSAID use (meloxicam), and 1 (1%) for decreasing aspirin dosage from 325 to 81 mg/d. The NSAIDs that were discontinued included ibuprofen, indomethacin, meloxicam, and naproxen.
Discussion
This project is the first, to the authors’ knowledge, to evaluate pharmacist interventions to reduce modifiable bleeding risk factors identified by the HAS-BLED bleeding risk score. Most of the patients with nonvalvular AF in the PC clinics did not have modifiable bleeding risk factors. However, of the patients who received a recommendation to reduce a specific modifiable risk factor, most of the interventions were accepted by PCPs and
Most of the interventions recommended evaluating the use of antiplatelet agents, particularly aspirin. The benefits of antiplatelet therapy for secondary prevention of cardiovascular disease are well established. However, for AF patients on OAC therapy, the concomitant use of antiplatelet therapy significantly increases the risk of bleeding and should be reserved for high-risk patients.10 The 2012 American College of Chest Physicians guidelines support the use of OAC monotherapy in patients with AF with stable CAD, including patients with a myocardial infarction or percutaneous coronary intervention more than 1 year previously, which has been corroborated with guideline and expert consensus recommendations released in 2016.7,8,10,11
For patients taking warfarin for AF without CAD, the possible benefit of concomitant aspirin therapy for primary prevention is outweighed by the increased risk of major bleeding.12 Furthermore, warfarin monotherapy has been shown to be effective in primary prevention of CAD and seems to have cardiovascular benefit for secondary prevention but with increased bleeding.13,14 As a result, through the exclusion criteria this project aimed to evaluate warfarin patients with AF at low risk of cardiovascular events who might be taking unnecessary concurrent antiplatelet therapy.
More than one-half of the interventions involved discontinuing antiplatelet therapy. Chart reviews revealed a lack of documentation for the indication and intended duration of antiplatelet therapy. In many patients, it is likely that aspirin use predated AF diagnosis and warfarin initiation. In some of these cases, it would have been appropriate to discontinue aspirin when starting warfarin use. Although there is guidance to support the use of OAC monotherapy in patients with AF with stable CAD, the patient’s provider and cardiologist made the decision to discontinue an antiplatelet agent after weighing benefits and risks. Regardless of the outcome, this analysis revealed the importance and need for routine review of antiplatelet therapy and documenting the rationale for antiplatelet use in addition to anticoagulation.
The second largest category of interventions accepted was for evaluation of NSAID use. A 2014 study by Lamberts and colleagues found that concomitant use of oral anticoagulants and NSAIDs conferred an independent risk for major bleeding and thromboembolism in patients with AF.15 The increase in serious bleeding (absolute risk difference of 2.5 events per 1,000 patients) was observed even with short-term NSAID exposure of 14 days across all NSAID types (selective COX-2 inhibitors or nonselective NSAIDs). In addition, there was an incremental increase in bleeding risk with high NSAID dosages. The risk of serious bleeding was even greater when an NSAID is added to OAC therapy and aspirin. Seven out of 17 warfarin patients (41%) who were taking an NSAID also were on an antiplatelet agent. As a result of the pharmacist interventions, NSAIDs were discontinued in all of these patients, but the antiplatelet remained because of stent placement or carotid stenosis.
This analysis captured only those patients with a documented active prescription or self-reported OTC use of an NSAID. It is unknown how many patients might take OTC NSAIDs occasionally but not report this use to a provider or pharmacist. Primary care CPSs educate patients to not use NSAIDs while taking warfarin during their initial visit and periodically thereafter; however, with the number of different NSAIDs available without a prescription and the various brand and generic names offered, it can be difficult for patients to understand what they should or should not take for minor pain or fever. Therefore, it is imperative that NSAID use is reviewed regularly at anticoagulant follow-up visits and patients are educated about alternative OTC agents for pain relief (eg, acetaminophen, topical agents, heating pad) when necessary. It also is equally important for PCPs to weigh the benefit vs risk for each patient before prescribing an NSAID if alternatives have been exhausted especially if the patient also is taking an OAC and antiplatelet agent.
The smallest number of interventions completed was for BP management. According to the HAS-BLED bleeding risk score, BP management was recommended only if the most recent clinic SBP was > 160 mm Hg, excluding patients with documented white-coat syndrome or home SBP readings < 160 mm Hg. One potential explanation for the small number of patients with SBP > 160 mm Hg is that for many of the patients taking warfarin, the PC CPSs at CJZVAMC have been involved in their BP management through earlier consultation by providers.
Limitations
A limitation of the BP component of the HAS-BLED score was that the assessment of BP for this project was only one point in time. In 3 cases, the SBP was > 160 mm Hg only during the most recent measurement, and these patients had normal BP readings on return to the clinic for follow-up. This category of recommendation also took more time for follow-up because a PC CPS would need to evaluate the patient in clinic, implement changes to BP medications, and follow-up at subsequent visits. Although some PCPs felt that the patient did not need pharmacist intervention, the elevated SBPs were brought to the provider’s attention, and some patients received further monitoring by the PCP or through a specialty clinic (eg, nephrology).
Another limitation of this project was that the bleeding risk evaluation occurred at only 1 visit. Patients’ medications and medical issues often change with time. Therefore, it is important to implement a process to regularly review (eg, annually) patients’ bleeding risk factors and to identify and act on modifiable risk factors. Another limitation was a lack of a comparator group and the time frame of the evaluation. As a result, the authors were unable to evaluate bleeding outcomes because of the small sample size and limited time frame. Future studies could consider evaluating bleeding events as an outcome, including additional modifiable risk factors, such as excess alcohol and labile INR, expanding the review to patients taking warfarin for indications other than AF, and review of patients on direct-acting oral anticoagulants (DOACs) with AF; keeping in mind that currently available bleeding risk calculators were developed for patients taking warfarin, not DOACs with AF. Patients could be counselled on reducing alcohol intake or switching to a DOAC if INR is labile despite adherence.
Conclusion
This quality improvement project successfully implemented use of the HAS-BLED bleeding risk score to identify and reduce modifiable bleeding risk factors in patients with AF taking warfarin. Pharmacist intervention resulted in a reduction of HAS-BLED scores and bleeding risk categories.
Click here to read the digital edition.
1. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042-1046.
2. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371-2379.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
4. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
5. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60(9):861-867.
6. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
7. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
8. Ruff CT, Ansell JE, Becker RC, et al. North American thrombosis forum, AF action initiative consensus document. Am J Med. 2016;129(suppl 5):S1-S29.
9. Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728-738.
10. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation. Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
11. Macle L, Cairns J, Leblanc K, et al; CCS Atrial Fibrillation Guidelines Committee. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170-1185.
12. Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med. 2007;167(2):117-124.
13. The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351(9098):233-241.
14. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
15. Lamberts M, Lip GY, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698.
1. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042-1046.
2. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371-2379.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
4. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
5. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60(9):861-867.
6. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
7. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
8. Ruff CT, Ansell JE, Becker RC, et al. North American thrombosis forum, AF action initiative consensus document. Am J Med. 2016;129(suppl 5):S1-S29.
9. Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728-738.
10. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation. Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e531S-e575S.
11. Macle L, Cairns J, Leblanc K, et al; CCS Atrial Fibrillation Guidelines Committee. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32(10):1170-1185.
12. Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med. 2007;167(2):117-124.
13. The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351(9098):233-241.
14. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969-974.
15. Lamberts M, Lip GY, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698.