User login
When and why to initiate antipsychotic polypharmacy, and with which agents
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
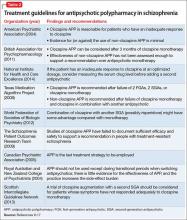
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
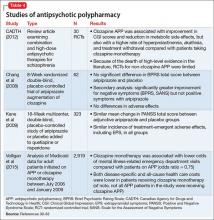
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
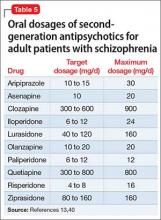
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.
When to initiate
Most treatment guidelines9-17 recommend clozapine only after monotherapy with at least 2 other antipsychotics fails. It is reasonable to add an antipsychotic to clozapine in patients who have shown a partial response to clozapine after a minimum of 3 months. Non-clozapine APP should be considered when:
- a patient derives no benefit from clozapine
- refuses clozapine
- clozapine is contraindicated
- APP is initiated to mitigate side effects from another antipsychotic.
Antipsychotics could take up to 16 weeks to achieve full efficacy,6 therefore, an adequate trial period within the target dosage range is advised for all antipsychotics (Table 5).13,40
Why initiate
Based on available data, partial response to maximum recommended dosages of antipsychotic monotherapy, including clozapine, or inability to tolerate higher dosages, provides a reason for initiating APP. Non-clozapine APP generally should be considered only in patients who refuse, cannot tolerate, or do not respond to clozapine. Consider using validated rating scales to track treatment outcomes (ideally, a ≥20% symptomatic reduction on the BPRS or PANSS), although there is no formal guidance regarding their use or benefit in APP.
Summing up
APP is a fairly common prescribing practice, even though safety and efficacy data are mixed. The issue of APP has become prevalent enough that regulatory bodies are involved in its monitoring and documentation.41
Clozapine APP, especially with risperidone, has the most substantial evidence to support it. Although APP generally is well tolerated, the overall dearth of conclusive safety and efficacy data indicates that this practice should be reserved for patients who have not responded adequately to monotherapy, including clozapine. Adjunctive aripiprazole could be considered for addressing symptomatic hyperprolactinemia or other metabolic effects caused by other antipsychotics.
An adequate trial as long as 16 weeks is advised before assessing the efficacy of any antipsychotic regimen. If APP provides inadequate response, or if there is no clear indication for APP, consider switching the patient back to monotherapy.42-44
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
2. Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527-533.
3. Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71(9):728-738.
4. Tapp A, Wood AE, Secrest L, et al. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55-59.
5. Ananth J, Parameswaran S, Gunatilake S. Antipsychotic polypharmacy. Curr Pharm Des. 2004;10(18):2231-2238.
6. Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321-322.
7. Botts S, Hines H, Littrell R. Antipsychotic polypharmacy in the ambulatory care setting, 1993-2000. Psychiatr Serv. 2003;54(8):1086.
8. Santone G, Bellantuono C, Rucci P, et al. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiol Drug Saf. 2011;20(5):441-449.
9. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Updated September 2009. Accessed September 20, 2014.
10. Barnes TRE; Schizophrenia Consensus Group of the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. http://www.bap.org.uk/pdfs/Schizophrenia_Consensus_Guideline_Document.pdf. Updated 2011. Accessed September 20, 2014.
11. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. http://www.nice.org.uk/guidance/cg178. Published February 2014. Accessed September 20, 2014.
12. Texas Medication Algorithm Project. Schizophrenia treatment algorithms. http://www.jpshealthnet.org/sites/default/files/tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed September 20, 2014.
13. Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
14. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. https://ww1.cpa-apc.org/Publications/Clinical_Guidelines/schizophrenia/november2005/index.asp. Updated November 2005. Accessed February 26, 2016.
15. Royal Australian and New Zealand College of Psychiatrists. Clinical practice guidelines for the treatment of schizophrenia and related disorders. http://www.ranzcp.org/Files/ranzcp-attachments/Resources/Publications/CPG/Clinician/CPG_Clinician_Full_Schizophrenia-pdf.aspx. Updated May 2005. Accessed February 26, 2016.
16. Scottish Intercollegiate Guidelines Network. Management of schizophrenia: a national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/131/index.html. Updated March 2013. Accessed September 20, 2014.
17. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
18. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661-681.
19. Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7-20.
20. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
21. Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27(4):327-336.
22. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1-11.
23. Akdede BB, Anil Ya˘gcio˘glu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919.
24. Anil Ya˘gcio˘glu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72.
25. Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94.
26. Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482.
27. Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136.
28. Weiner E, Conley RR, Ball MP, et al. Adjunctive risperidone for partially responsive people with schizophrenia treated with clozapine. Neuropsychopharmacology. 2010;35(11):2274-2283.
29. Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomized controlled clinical trial. J Psychopharmacol. 2009;23(3):305-314.
30. Canadian Agency for Drugs and Technology in Health. Current utilization of antipsychotic agents for schizophrenia: combination and high-dose therapies. https://www.cadth.ca/sites/default/files/pdf/H0503_AAP-Current-Utilization-Report_e.pdf. Published August 2012. Accessed February 26, 2016.
31. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine treated patients with refractory schizophrenia: an 8-week, randomized, double blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731.
32. Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348-1357.
33. Velligan DI, Carroll C, Lage MJ, et al. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66(2):127-133.
34. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
35. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
36. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
37. Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404-1410.
38. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand. 2013;127(3):217-226.
39. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;26(2):165-169.
40. Drug Information Handbook, 22th ed. Hudson, OH: Lexi-Comp, Inc.; 2013:1143-1147.
41. The Joint Commission. Specifications Manual for Joint Commission National Quality Measures (v2013A1). https://manual.jointcommission.org/releases/TJC2013A/. Accessed on May 13, 2015.
42. Essock SM, Schooler NR, Stroup TS, et al; Schizophrenia Trials Network. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702-708.
43. Godleski LS, Kerler R, Barber JW, et al. Multiple versus single antipsychotic drug treatment in chronic psychosis. J Nerv Ment Dis. 1989;177(11):686-689.
44. Suzuki T, Uchida H, Tanaka KF, et al. Revising polypharmacy to a single antipsychotic regimen for patients with chronic schizophrenia. Int J Neuropsychopharmacol. 2004;7(2):133-142.