User login
Targeting diabetes: The benefits of an integrative approach
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
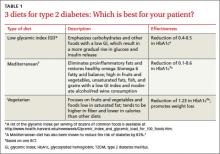
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; jackie.redmer@fammed.wisc.edu
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; jackie.redmer@fammed.wisc.edu
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
- Tell patients with type 2 diabetes mellitus (T2DM) that chromium and fiber appear to have a beneficial effect on glycemic control, while the benefits of other dietary supplements are not known. C
- Recommend acupuncture for patients with T2DM and peripheral neuropathy, bladder dysfunction, or symptoms of other comorbidities that have not fully responded to conventional therapy. B
- Advise patients that biofeedback and meditation are more likely than other types of stress reduction to improve glycemic control. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Integrative medicine—an approach that combines conventional and alternative therapies with an emphasis on natural, less invasive evidence-based options—is well suited to the management of complex chronic diseases like type 2 diabetes mellitus (T2DM). And we’ve found that patients with T2DM are increasingly interested in integrative strategies, most of which involve self-management and lifestyle changes. They’re often motivated by the desire to limit the number of medications they’re taking or to avoid diabetes drugs entirely. In many cases, patients also hope to alleviate symptoms of comorbidities that have not fully responded to conventional treatment, such as peripheral neuropathy, bladder dysfunction, and gastroparesis.
As a family physician, you’re likely to be asked about unconventional approaches to diabetes and to be in a position to recommend alternative therapies in conjunction with pharmaceutical management of T2DM. In both cases, you need to know which integrative strategies have evidence to support their use. We created the text and tables that follow with this in mind.
Nutrition and weight loss: What works?
To reduce cardiovascular disease risk factors, patients with T2DM are advised to eliminate trans fats and to limit saturated fat to <7% of total caloric intake. A moderate weight loss (5% of body weight) has been found to improve insulin action, decrease fasting blood glucose (FBG) concentrations, and reduce the need for diabetes medications.1,2 One small retrospective cohort study (N=72) found that a 10% weight loss was associated with a reduction in glycosylated hemoglobin (HbA1c) of 0.81 percentage points.3 Weight maintenance is also an important element of diabetes management, even for patients who have not been able to lose their excess weight.2
American Diabetes Association (ADA) guidelines do not endorse a specific diet. But patients often ask for dietary advice and want to know how specific diet plans and food choices will affect glycemic control and comorbid conditions. You can present the evidence (TABLE 1)4-6 of the effectiveness of a low glycemic index (GI) diet, a Mediterranean diet, and a vegetarian diet and point out that the best diet for a particular patient is the one best suited to his or her lifestyle and dietary goals.
Low glycemic index diet. The 2013 ADA guidelines acknowledge that a dietary regimen with a low glycemic index (GI) may be more beneficial for glycemic control than a diet based on total carbohydrate count alone.7
A Cochrane review of 11 small studies found a reduction in HbA1c of 0.5 (95% confidence interval [CI], -0.9 to -0.1; P=.02) in patients with T2DM who followed a low GI diet for 4 weeks or more. Patients on this diet also had a significant reduction in hypoglycemic events compared with those on other dietary regimens;4 one of the studies included in the meta-analysis also found a statistically significant increase in high-density lipoprotein cholesterol (HDL-C) in those on the low
GI diet.8
Mediterranean diet. Rich in fruits and vegetables and unsaturated fats, fish, and grains with a low GI, the Mediterranean diet has a higher carbohydrate and fat content than a portion-controlled diet formerly recommended by the ADA. (As noted earlier, current ADA guidelines do not recommend a particular diet).
A systematic review of 5 randomized controlled trials (RCTs) (N=1077) found improved glycemic control in patients on a Mediterranean diet compared with those on other commonly used diets, such as low fat and portion-controlled regimens. Fasting blood glucose (FBG) fell between 7 and 40 mg/dL and HbA1c by 0.1 to 0.6 in studies that ranged from 6 months to 2 years.5 The effectiveness of the Mediterranean diet, despite its higher carbohydrate content, suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia. The effectiveness of the Mediterranean diet--despite its higher carbohydrate content--suggests that treating systemic inflammation may help to reduce insulin resistance and hyperglycemia.
Vegetarian diet. Vegetarian and vegan diets also offer potential benefits in the management of T2DM—including the fact that they tend to be lower in calories than other dietary regimens and therefore more likely to promote weight loss. In one 22-week RCT (N=99), those who were randomized to a low-fat vegan diet lost more weight (6.5 vs 3.1 kg; P<.001) and had a larger decrease in HbA1c (-1.23 vs -0.38; P<.01) and low density lipoprotein cholesterol (LDL-C) (-22.6 vs -10.7 mg/dL; P=.02) compared with those following a portion-controlled ADA diet. Glycemic change correlated with the change in body weight.6
Choose carefully among the supplements
Metformin was derived from Galega officinalis—a plant (sometimes called goat’s rue, or French lilac) used by Europeans as a traditional treatment for diabetes since the Middle Ages.9 More than 400 dietary supplements have been reported to have beneficial effects for patients with diabetes, including plant-based products such as fenugreek, prickly pear, and ginseng; vitamins, and minerals. But in most cases, the evidence is of poor quality (TABLE 2). Only one—cinnamon—has Level I evidence (ie, evidence derived from at least one well-designed RCT).
Two recent meta-analyses of cinnamon supplementation for patients with diabetes—one from 2012 and the other from 2011— yielded different results. The 2012 evidence comes from a Cochrane review showing that 3 types of cinnamon—true cinnamon (Cinnamomum zeylanicum), Chinese cinnamon (C cassia), and Indonesian cinnamon (C burmanni)—had no effect on HbA1c in patients with type 1 or T2DM and a borderline effect on FBG.10 However, the 2011 meta-analysis found that cinnamon supplementation led to a significant improvement in FBG.11
The bottom line? The evidence is inconclusive.
Fiber and chromium stand out
Fiber. Whether taken as a supplement or in foods such as chickpeas, beans, peas, and lentils, fiber has been shown to improve glycemic control.12,13 The ADA recommends 25 to 35 g of dietary fiber daily.13 The American Diabetes Association recommends 25 to 35 g of dietary fiber daily.
Chromium, a trace mineral thought to be a necessary cofactor for insulin regulation and glucose metabolism, is present in many foods, especially brewer’s yeast, liver, carrots, potatoes, broccoli, and spinach. Notably, however, refining grains and processing foods removes most absorbable chromium. Patients with T2DM may therefore benefit from chromium supplementation: A 2007 systematic review found that it lowered their HbA1c by an average of 0.6.14
What about other supplements?
The ADA does not generally support the use of micronutrient supplements for patients with diabetes, but notes that individuals at increased risk for deficiencies (eg, those following very-low-calorie diets, the elderly, and strict vegetarians) may benefit from multivitamin supplements.
Magnesium. Dietary sources of magnesium, which is involved in insulin secretion, binding, and activity, include whole grains, leafy green vegetables, legumes, and nuts. A deficiency is associated with decreased absorption (in patients with diets high in processed food, for example) or increased elimination (in those who ingest large quantities of alcohol or caffeine or take diuretics or birth control pills). A 2006 meta-analysis found that magnesium supplements led to an improvement in FBG, but did not significantly lower HbA1c in patients with T2DM.15
Vitamin D. Although individuals with the highest vitamin D levels (>25 ng/mL) have a 43% lower risk of developing diabetes,16 it is not known whether vitamin D supplements are beneficial to patients with T2DM. (For more on vitamin D supplementation, see the Practice Alert in this issue.)
Vitamin E. A fat-soluble antioxidant found in vegetable oil, nuts, and green leafy vegetables, vitamin E’s best-studied component is alpha-tocopherol. A 2011 meta-analysis found vitamin E supplementation to have a beneficial effect in patients with T2DM—but only for the subset of patients who had both low serum vitamin E levels and an HbA1c >8.0%.17 Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
Observational studies have raised questions about the safety of vitamin E supplements, and a 2005 meta-analysis concluded that high-dose vitamin E supplementation (≥400 IU/d) may increase all-cause mortality.18
What about omega-3 PUFAs?
The role of omega-3 polyunsaturated fatty acids (PUFAs) in the prevention and treatment of diseases related to inflammation has garnered much attention for years. But there is no evidence that taking omega-3 PUFAs lowers the risk of macrovascular outcomes or mortality for patients with T2DM.
Alpha lipoic acid (ALA), an antioxidant made by the body and found in very small amounts in foods, is widely used in Europe, and has shown promise in the treatment of diabetic neuropathy. Small studies have found that ALA may reduce oxidative stress and improve insulin sensitivity in patients with diabetes,19 and a recent small RCT showed a statistically significant decrease in FBG and postprandial glucose after 8 weeks of taking it.20
How to get patients moving
The ADA recommends that patients with T2DM get 150 minutes per week of moderate intensity aerobic activity (at 50%-70% of maximum heart rate), spread over at least 3 days a week and with no more than 2 consecutive days without activity. Resistance training provides additional benefit; the ADA recommends that patients engage in resistance training at least twice a week, using 5 major muscle groups.7 Regular exercise helps both weight reduction and glucose uptake, but simply pointing that out to patients with T2DM is not enough.
What works? A large meta-analysis found that structured exercise training (in most studies, this consisted of 2-5 supervised sessions weekly for 12-16 weeks) led to a decrease in HbA1c (-0.67; 95% CI, -0.84 to -0.49). When the structured exercise was aerobic, HbA1c declined by 0.73 (95% CI, -1.06 to -0.40); when it was resistance training, HbA1c fell by 0.57 (95% CI, -1.14 to -0.01).21 Simply advising a patient to be physically active—without involvement in both the planning and supervision—led to statistically significant reductions in HbA1c only when the advice was combined with dietary recommendations. Giving patients both exercise advice and dietary recommendations led to an HbA1c reduction of 0.58 (95% CI, -0.74 to -0.43).21
The duration of the structured exercise mattered, too, of course, with those who exercised more than 150 minutes per week achieving a larger reduction in HbA1c than those who exercised 150 minutes or less (-0.89; 95% CI, -1.26 to -0.50 vs -0.36; 95% CI, -0.50 to -0.23). Higher intensity activity did not improve glycemic For patients with type 2 diabetes, higher intensity activity does not appear to lead to greater improvement in glycemic control compared with moderate intensity activity. control any more than moderate intensity exercise.21
Mind-body stress relievers
The National Health Interview Survey (NHIS) estimated that in 2007 (the most recent survey that addressed mind-body modalities), 19% of US adults used at least one mind-body modality in the previous 12 months.22 Modalities included in the NHIS were biofeedback and yoga, body interventions best studied for diabetes management. Here’s what the evidence shows:
Biofeedback. In a small RCT (N=30 patients with T2DM) comparing biofeedback-assisted relaxation training (10 weekly 45-minute sessions) with education alone, the treatment group had significant improvement in HbA1c levels (which went from 7.4% to 6.8%) and in average blood glucose values that persisted at 3-month follow-up.23
Biofeedback can also produce clinically significant toe temperature elevations. In patients with T2DM, volitional warming has been associated with increased circulation, improvement or elimination of intermittent claudication pain, more rapid healing of diabetic ulcers, and improved functional status.24
Yoga. Two systematic reviews concluded that yoga is likely to benefit patients with T2DM, leading to lower blood sugar, LDL-C levels, triglycerides, body weight, waist-to hip ratio, and HbA1c, and higher HDL-C.25,26 Additionally, yoga appears to have a beneficial effect on the blood pressure, heart rate, oxidative stress, sympathetic activation, catecholamine levels, coronary stenosis, coagulation profiles, and pulmonary function of patients with T2DM, and is associated with reductions in the amount of medication needed and in psychosocial risk factors. (Because of the heterogeneous nature of the studies reviewed, however, no statistical analyses were reported.)
A third systematic review, which included only 5 studies, found that yoga yielded a short-term improvement in FBG and lipids, but no statistically significant improvement in long-term outcomes of body mass index, body weight, or HbA1c.27 All 5 studies noted that there were methodological problems and uncertainty about the generalizability of the findings to Western culture.
Meditation. The regular practice of transcendental meditation (TM) is associated with a reduction of catecholamine levels, a study comparing meditators with a control group found.28 A study examining the relationship between depression and diabetes found compelling evidence of an association between mental stress and hypothalamic-pituitary-adrenal axis hyperactivity,29and another comparing meditators with controls found the regular practice of TM to be associated with a reduction in catecholamine levels.30 As increased catecholamine levels affect glucose transport and insulin resistance, this finding suggests that reducing stress levels through meditation might lead to improved glycemic control. Transcendental meditation has been found to reduce mean arterial pressure, insulin resistance, and insulin levels.
One RCT comparing diabetes education alone with education plus stress management (progressive muscle relaxation, deep breathing, and mental imagery) found that HbA1c levels decreased by 0.5 in the latter group at one year.31In a single blinded randomized study, the TM group had a statistically significant reduction in mean arterial pressure, insulin resistance, and insulin levels compared with those who received diabetes education alone.32
Qigong. The effectiveness of Qigong systems such as Tai Chi—which integrate physical postures, breathing techniques, and focused attention—is difficult to determine because of methodological challenges in design and variability in practice. Authors of a systematic review of Tai Chi and diabetes found only 2 RCTs and 3 nonrandomized clinical trials and concluded that there was no convincing evidence that the practice aids in glucose control.33 Two other systematic reviews of Qigong for T2DM reported some improvement in glucose control, but limited study quality prevented definite
conclusions.34,35
When to consider "manual medicine"
An integrative approach to health also includes a number of modalities collectively known as manual medicine: acupuncture, massage/energy therapy, acupressure, and chiropractic and other forms of manipulation. Evidence on these modalities for the treatment of diabetes and diabetic complications is limited.
Acupuncture. Although acupuncture has long been reported to improve glycemic control in patients with diabetes and prediabetes, the evidence is limited and of poor quality.36,37
In recent years, 2 small RCTs have found that acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol.38,39 In one of the studies, 87.5% of participants randomized to acupuncture had symptom improvement, compared with 63.6% of those in the oral inositol group. In fact, marked symptom relief after 3 months of treatment was reported by 50% of those Acupuncture reduced pain in patients with diabetic peripheral neuropathy vs sham acupuncture or oral inositol. who had acupuncture, compared with 21% of those who did not.39
In a small 2-week RCT, patients randomized to acupuncture vs sham acupuncture for diabetic bladder dysfunction showed statistically significant improvements in both subjective symptoms and urodynamic measurements.40 And a study comparing patients receiving electroacupuncture—in which an electric current is transmitted between 2 needles placed in the muscles—vs sham acupuncture found nonstatistically significant improvements in symptomatic
gastroparesis.41
Massage/energy therapy. Massage has been shown in several studies to reduce glucose levels,42-44 although no reductions in glucose levels were found in one small RCT.45 Connective tissue reflex massage led to improved lower limb blood flow in patients with diabetes and peripheral artery disease in another study, but the clinical significance is uncertain.46 Studies of reflexology and acupressure are similarly limited to small experimental and observational studies.47 No RCTs of chiropractic treatment for diabetes were found.
CORRESPONDENCE
Jacqueline Redmer, MD, MPH, Department of Family Medicine, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; jackie.redmer@fammed.wisc.edu
ACKNOWLEDGEMENTS
The authors thank Drs. Sarina Schrager and Mindy Smith for their manuscript assistance. The work presented here was carried out while Drs. Longmier and Wedel were Primary Care Research Fellows supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.
1. Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26(suppl 1):S51–S61.
2. Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27:2067–2073.
3. Shantha GP, Kumar AA, Kahan S, et al. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417–426.
4. Thomas D, Elliott EJ. Low glycaemic index or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;(1):CD006296.
5. Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102.
6. Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783.
7. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66.
8. Jenkins DJA, Kendall CWC, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753.
9. Hadden DR. Goat’s rue-French lilac-Italian fitch-Spanish sainfoin: Gallega officinalis and metformin: the Edinburgh connection. J R Coll Physicians Edinb. 2005;35:258–260.
10. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
11. Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889.
12. Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16–23.
13. Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495.
14. Balk EM, Tatsioni A, Lichtenstein AH, et al. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163.
15. Song Y, He K, Levitan EB, et al. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes. Diabet Med. 2006;23:1050–1056.
16. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015.
17. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36:53–63.
18. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
19. Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9:392–398.
20. Ansar H, Mazloom Z, Kazemi F, et al. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32:584–588.
21. Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. JAMA. 2011;305:1790–1799.
22. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States 2007. Natl Health Stat Rep. 2008;(12):1–23.
23. McGinnis RA, McGrady A, Cox SA, et al. Biofeedback-assisted relaxation in type 2 diabetes. Diabetes Care. 2005;28:2145–2149.
24. Galper DI, Taylor AG, Cox DJ. Current status of mind-body interventions for vascular complications of diabetes. Fam Community Health. 2003;26:34–40.
25. Alexander GK, Taylor AG, Innes KE, et al. Contextualizing the effects of yoga therapy on diabetes management. Fam Community Health. 2008;31:228–239.
26. Innes KE, Vincent HK. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med. 2007;4:469–486.
27. Aljasir B, Bryson M, Al-Shehri B. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. Evid Based Complement Alternat Med. 2010;7:399–408.
28. Infante JR, Torres-Avisbal M, Pinel P, et al. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 2001;72:141–146.
29. Dusek JA, Benson H. Mind-body medicine: a model of the comparative clinical impact of the acute stress and relaxation responses. Minn Med. 2009;92:47–50.
30. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36:1276–1286.
31. Surwit RS, Van Tilburg MAL, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25:30–34.
32. Paul-Labrador M, Polk D, Dwyer JH, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch Intern Med. 2006;166:1218–1224.
33. Lee MS, Pittler MH, Kim M-S, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241.
34. Chen KW, Liu T, Zhang H, et al. An analytical review of the Chinese literature on Qigong therapy for diabetes mellitus. Am J Chin Med. 2009;37:439–457.
35. Lee MS, Chen KW, Choi T-Y, et al. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17:236–242.
36. Hu H. A review of treatment of diabetes by acupuncture during the past forty years. J Tradit Chin Med. 1995;15:145–154.
37. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes Obes Metab. 2010;12:555–569.
38. Tong Y, Guo H, Han B. Fifteen-day acupuncture treatment relieves diabetic peripheral neuropathy. J Acupunct Meridian Stud. 2010;3:95–103.
39. Zhang C, Ma Y-X, Yan Y. Clinical effects of acupuncture for diabetic peripheral neuropathy. J Tradit Chin Med. 2010;30:13–14.
40. Tong Y, Jia Q, Sun Y, et al. Acupuncture in the treatment of diabetic bladder dysfunction. J Alternat Complement Med. 2009;15:905–909.
41. Wang C-P, Kao C-H, Chen W-K, et al. A single-blinded, randomized pilot study evaluating effects of electroacupuncture in diabetic patients with symptoms suggestive of gastroparesis. J Alternat Complement Med. 2008;14:833–839.
42. Guthrie DW, Gamble M. Energy therapies and diabetes mellitus. Diabetes Spectr. 2001;14:149–153.
43. Ezzo J, Donner T, Nickols D, et al. Is massage useful in the management of diabetes? A systematic review. Diabetes Spectr. 2001;14:218–224.
44. Sajedi F, Kashaninia Z, Hoseinzadeh S, et al. How effective is Swedish massage on blood glucose level in children with diabetes mellitus? Acta Med Iran. 2011;49:592–597.
45. Wändell PE, Carlsson AC, Gåfvels C, et al. Measuring possible effect on health-related quality of life by tactile massage or relaxation in patients with type 2 diabetes. Complement Ther Med. 2012;20:8–15.
46. Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12.
47. Pilkington K, Stenhouse E, Kirkwood G, et al. Diabetes and complementary therapies: mapping the evidence. Pract Diab Int. 2007;24:371–376.