User login
Sweet Syndrome With Aseptic Splenic Abscesses and Multiple Myeloma
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
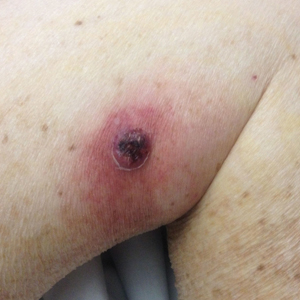
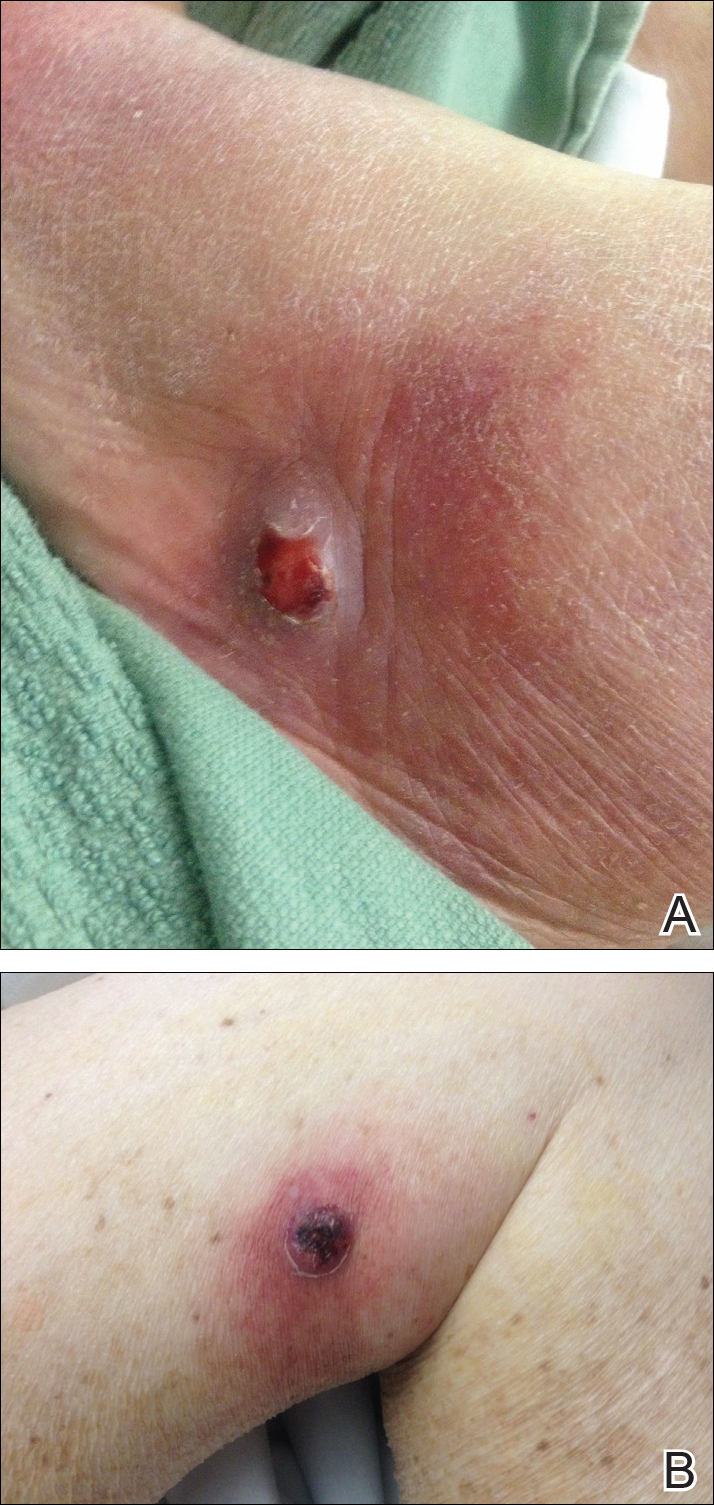
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.
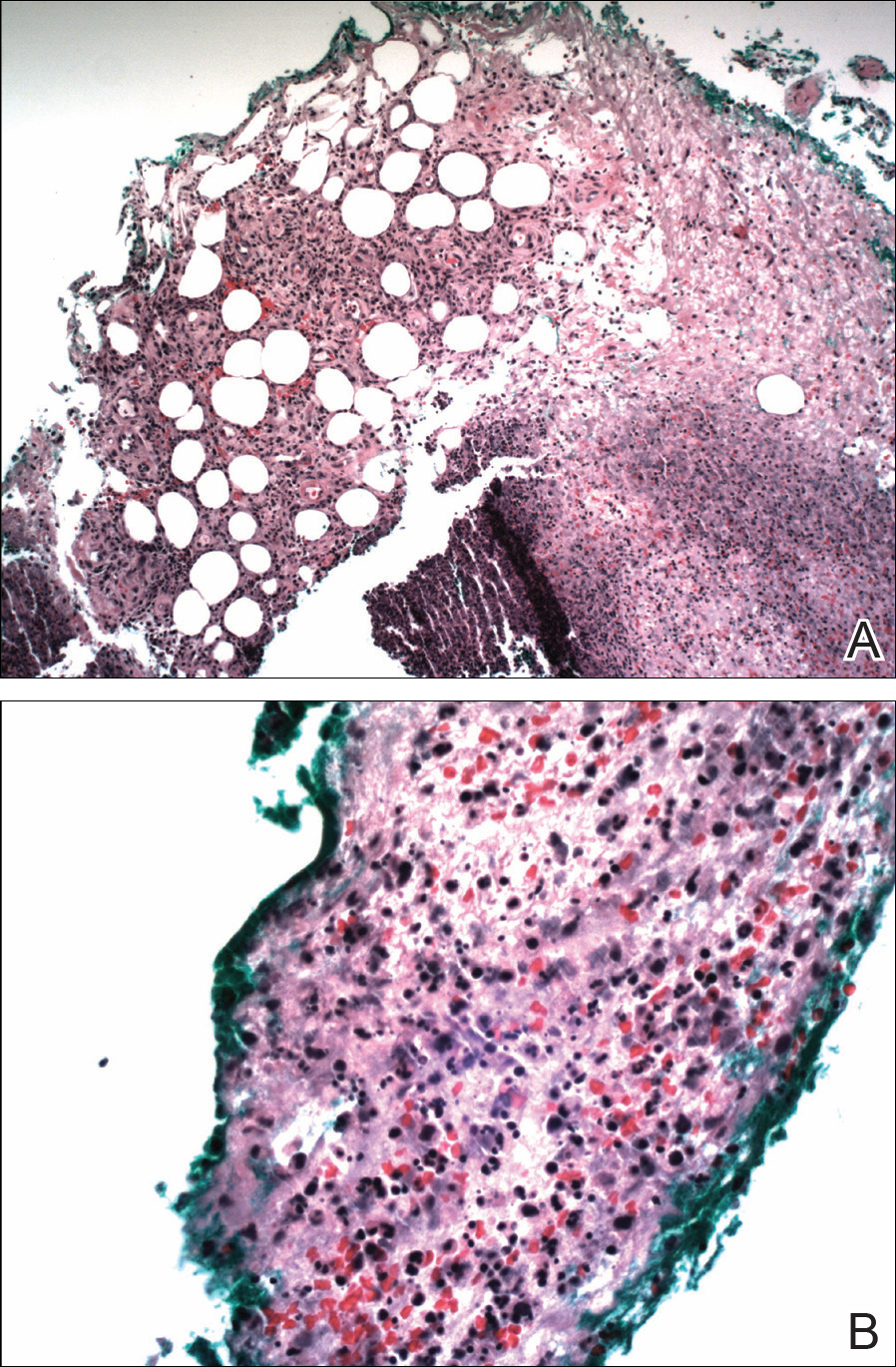
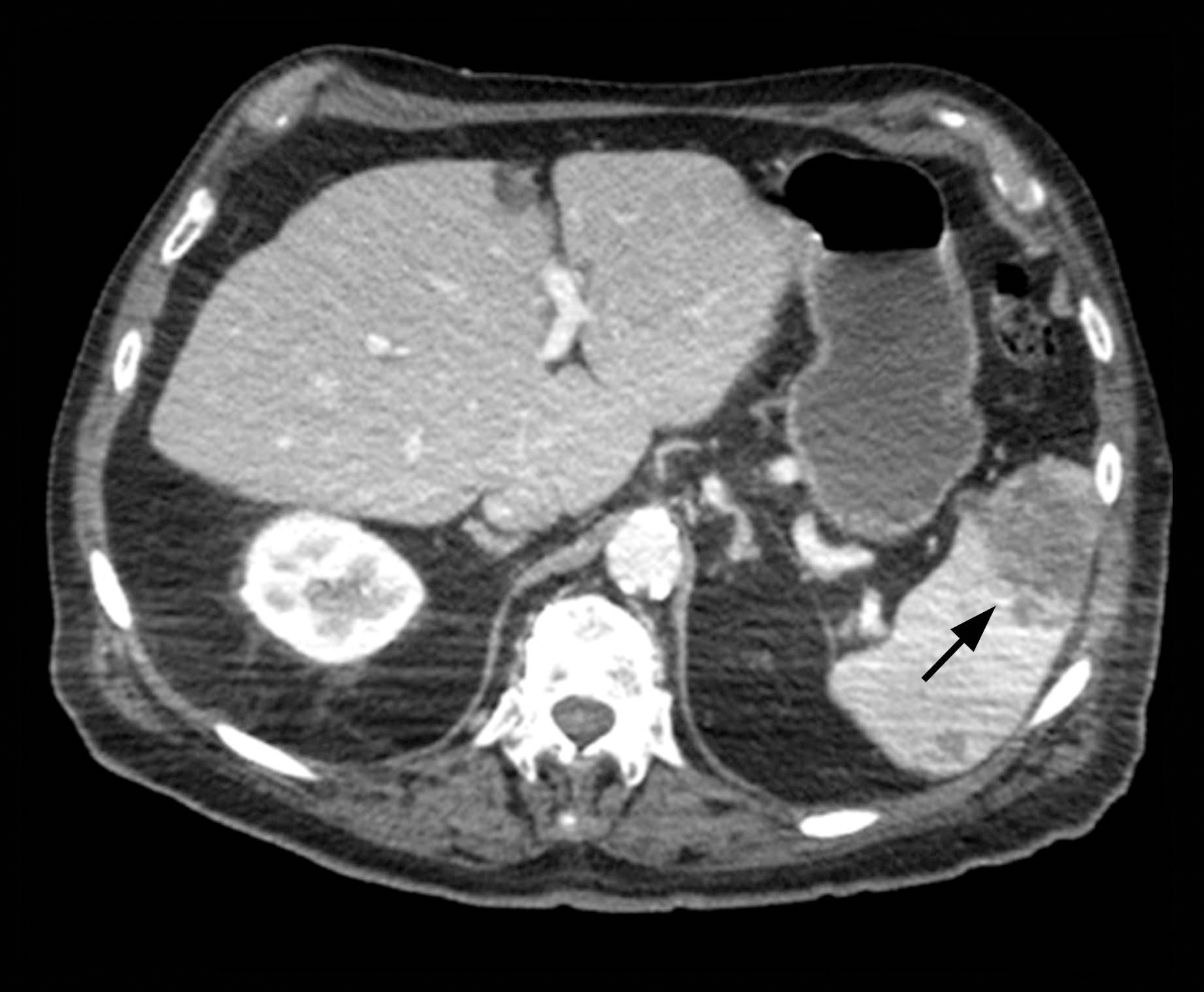
The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.
Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.

The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.


Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
To the Editor:
An 84-year-old man was admitted to the hospital with 5 erythematous cutaneous nodules of several days’ duration on the legs ranging in size from 1.0 to 1.5 cm. Upon admission, the patient also had a chest radiograph suspicious for pneumonia. The patient had received sulfamethoxazole/trimethoprim for a urinary tract infection as an outpatient 5 days prior to presentation, but he stopped the medication due to the appearance of the cutaneous nodules. Of note, the patient also reported unintentional weight loss of 15 pounds over the last few months.
New nodules had developed at a rate of 1 to 2 lesions daily in the 3 days prior to presentation and continued to develop after admission to the hospital. The nodules appeared as tender, erythematous lesions that evolved to form pustules and developed overlying crusts in later stages (Figure 1). They were limited to the arms and legs, primarily involving the lower legs. There was no evidence of oral or ocular involvement. A hemoglobin count of 10.9 g/dL (reference range, 14.0–17.5 g/dL), white blood cell count of 8.8×109/L (reference range, 4.5–11.0×109/L), and erythrocyte sedimentation rate of 69 mm/h (reference range, 0–20 mm/h) were noted on admission.

The patient was started on ceftriaxone and azithromycin for suspected pneumonia. The differential diagnosis for the cutaneous nodules included lymphoma, acid-fast bacilli (AFB) infection, deep fungal infection, pyoderma gangrenosum, Sweet syndrome (SS), panniculitis, erythema elevatum diutinum, and polyarteritis nodosa. A punch biopsy of a nodule on the left foot was performed. Histopathology demonstrated a neutrophilic panniculitis (Figure 2) with an epidermal abscess. No vasculitis was identified, and periodic acid–Schiff and AFB staining of the skin biopsy were negative. These findings were consistent with SS. Computed tomography scans of the chest, abdomen, and pelvis, which were completed early in the course of hospitalization due to concern for underlying malignancy, revealed pericardial and pleural effusions as well as cystic lesions in the lungs, spleen, kidneys, and prostate, with the largest lesion on the spleen measuring 5.6×4.8 cm (Figure 3). Computed tomography scanning was negative for areas of consolidation in the lungs. A splenic biopsy was performed by an interventional radiologist during the patient's hospitalization that identified an aseptic, neutrophilic process. Fungal, bacterial, and AFB cultures of the splenic tissue and cystic contents were negative. Bilateral pleural effusions also were identified, and a thoracentesis was performed. The pleural fluid indicated rare mesothelial cells in the background of acute inflammation with no growth of the bacterial, fungal, or AFB cultures.


Due to the association of hematologic malignances with SS, a bone marrow biopsy was performed, which revealed multiple myeloma. Serum protein electrophoresis demonstrated monoclonal gammopathy of κ light chains. During the course of his hospitalization, new skin lesions continued to develop on the hands, face, and trunk. The patient was discharged from the hospital shortly after diagnosis to receive outpatient treatment for multiple myeloma with lenalidomide and dexamethasone. Upon follow-up with the patient’s family via telephone 3 weeks into treatment, his son confirmed that the nodules were resolving.
Our case could be consistent with either drug-induced or malignancy-associated SS. Sweet syndrome initially was described in 1964 in 8 female patients with leukocytosis and cutaneous plaques infiltrated by neutrophils.1 The skin lesions typically are red and painful, ranging in size from 0.5 cm to 12.0 cm, and can last weeks to years if not treated.2 Variations of skin lesions include bullous and pustular morphologies.3
Diagnostic criteria for SS have been established.4 Both of the major criteria must be met as well as 2 of 4 minor criteria. Major criteria include abrupt onset of tender erythematous plaques and nodules; secondly, a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis must be seen on histopathology. Minor criteria include pyrexia, association with underlying condition (malignancy, pregnancy, drug exposure, inflammatory disorder), responsiveness to systemic steroids, and abnormal laboratory values (erythrocyte sedimentation rate, white blood cell count, C-reactive protein, neutrophilia).4
Sweet syndrome can be divided into 3 classifications: classical or idiopathic, drug-induced, or malignancy-associated.4 Classical SS most commonly is seen in middle-aged women after an upper respiratory or gastrointestinal infection. Drug-induced SS most often is associated with granulocyte-stimulating factor colony therapy4; however, it has been associated with use of trimethoprim/sulfamethoxazole.5 Malignancy-associated SS most commonly is seen in individuals with hematologic malignancy, specifically acute myeloid leukemia. Although its association with multiple myeloma is not as frequent, cases of malignancy-associated SS identifying this association have been reported.6,7 Mucosal involvement in the form of aphthouslike lesions more frequently is seen in malignancy-associated SS.8 Differing from classical SS, which has a female predilection of around 4:1, the malignancy-associated disorder has a 1:1 female-to-male ratio.4
In the majority of cases of SS, the neutrophilic infiltrate is in the papillary and upper reticular dermis; however, if the neutrophilic infiltrate is predominately in the subcutaneous tissue (known as subcutaneous SS), there is a strong association with malignancy.9 The histopathology in our case demonstrated a neutrophilic infiltrate in the subcutaneous tissue.
Fever is the most common systemic manifestation of SS and is present in 54% to 65% of patients.8,10 Besides the skin, the most common site affected is the eye, with 13% to 75% of patients reporting ocular involvement, usually conjunctivitis.4,10 Although infrequent, extracutaneous SS has been identified in the bones, central nervous system, kidneys, heart, liver, spleen, lungs, ears, eyes, and intestines.4 A case of SS with splenic involvement in the form of sterile abscesses also was reported.11 This case was related to parvovirus B19.
Sweet syndrome is a condition characterized by tender, erythematous cutaneous lesions with histopathology demonstrating neutrophilic infiltrate in the absence of vasculitis. We report a case of suspected extracutaneous SS in the form of splenic cysts in a patient whose SS was associated with malignancy and/or drug ingestion.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome and malignancy. Am J Med. 1987;82:1220-1226.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2002;41:182-184.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Belhadjali H, Chaabane S, Njim L, et al. Sweet’s syndrome associated with multiple myeloma. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:31-33.
- Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol. 2003;30:261-264.
- Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc. 1995;70:234-240.
- von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560.
- Neoh CY, Tan AW, Ng SK. Sweet’s syndrome: a spectrum of unusual clinical presentation and associations. Br J Dermatol. 2007;156:480-485.
- Fortna RR, Toporcer M, Elder DE, et al. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and review of the literature on extracutaneous Sweet syndrome. Am J Dermatopathol. 2010;32:621-627.
Practice Points
- Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an inflammatory process characterized by a diffuse dermal neutrophilic infiltrate in the absence of vasculitis.
- A diagnosis of SS warrants further investigation due to its association with malignancy, especially hematologic malignancy.
- Other organs in SS also may have aseptic involvement.
What’s Eating You? Tick Bite Alopecia
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later
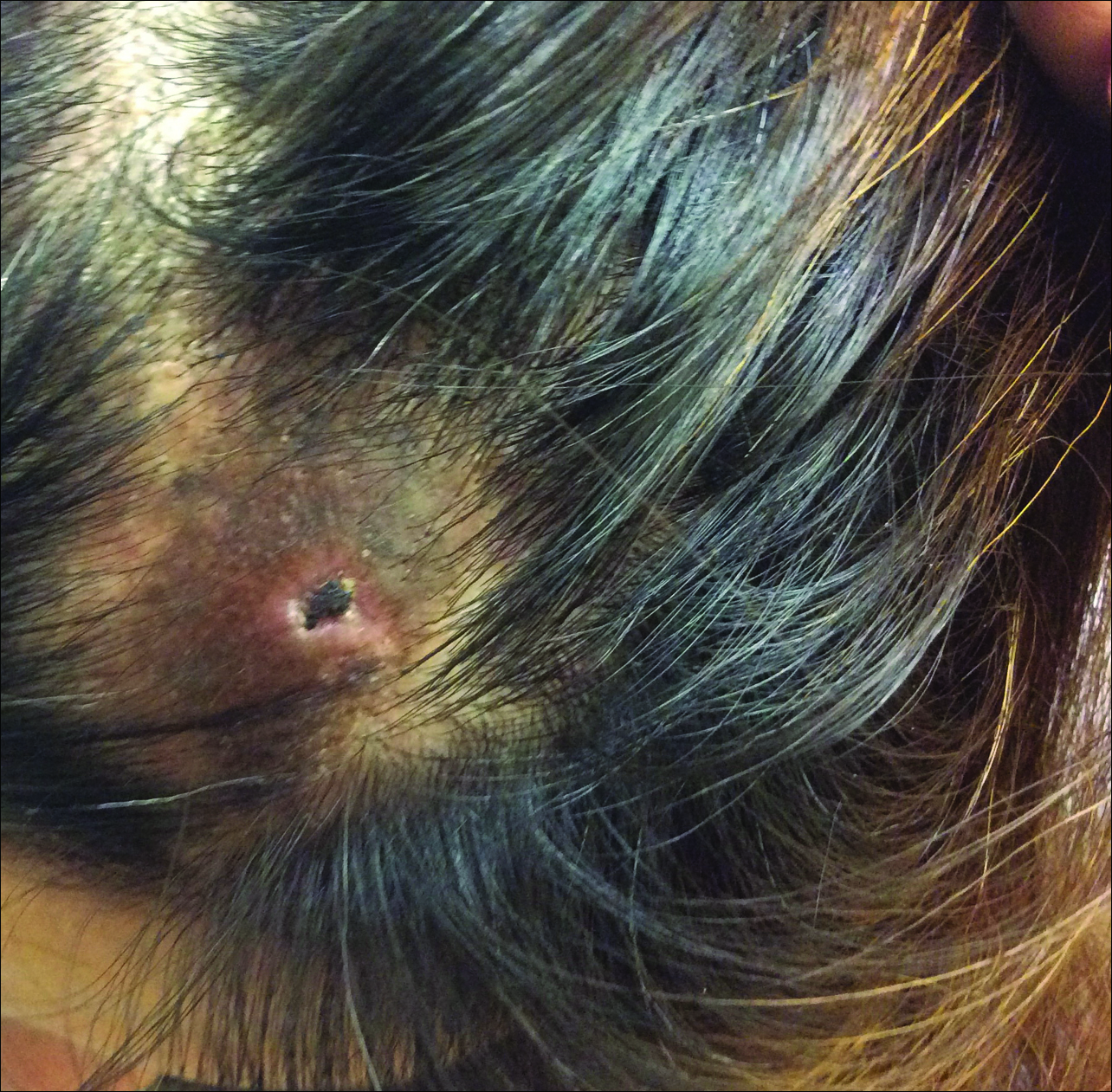
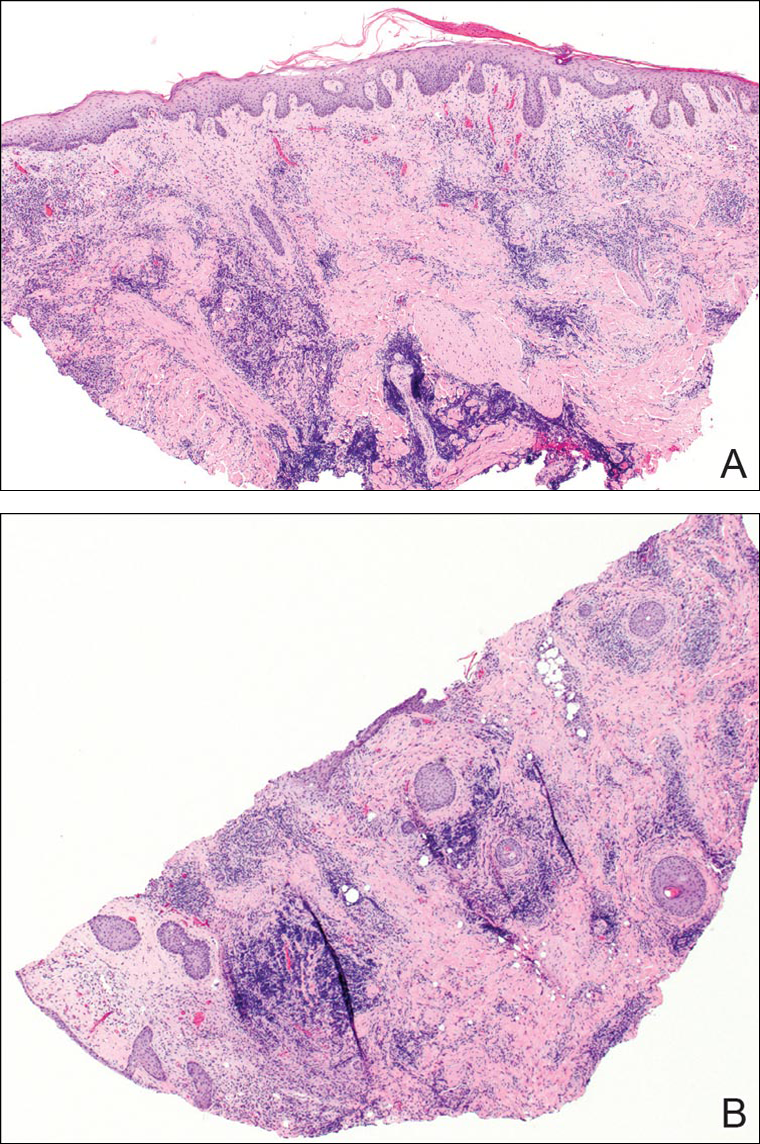
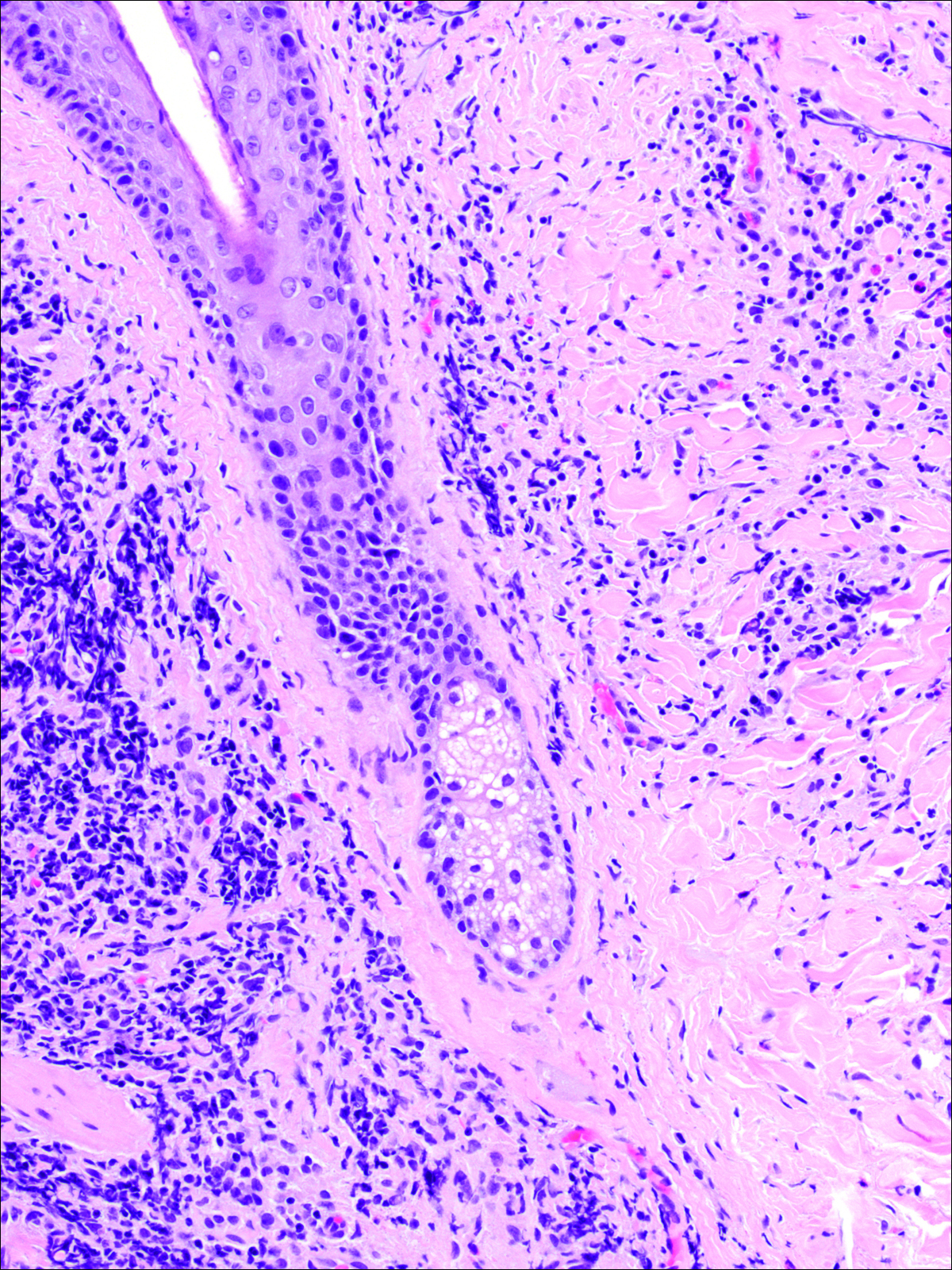
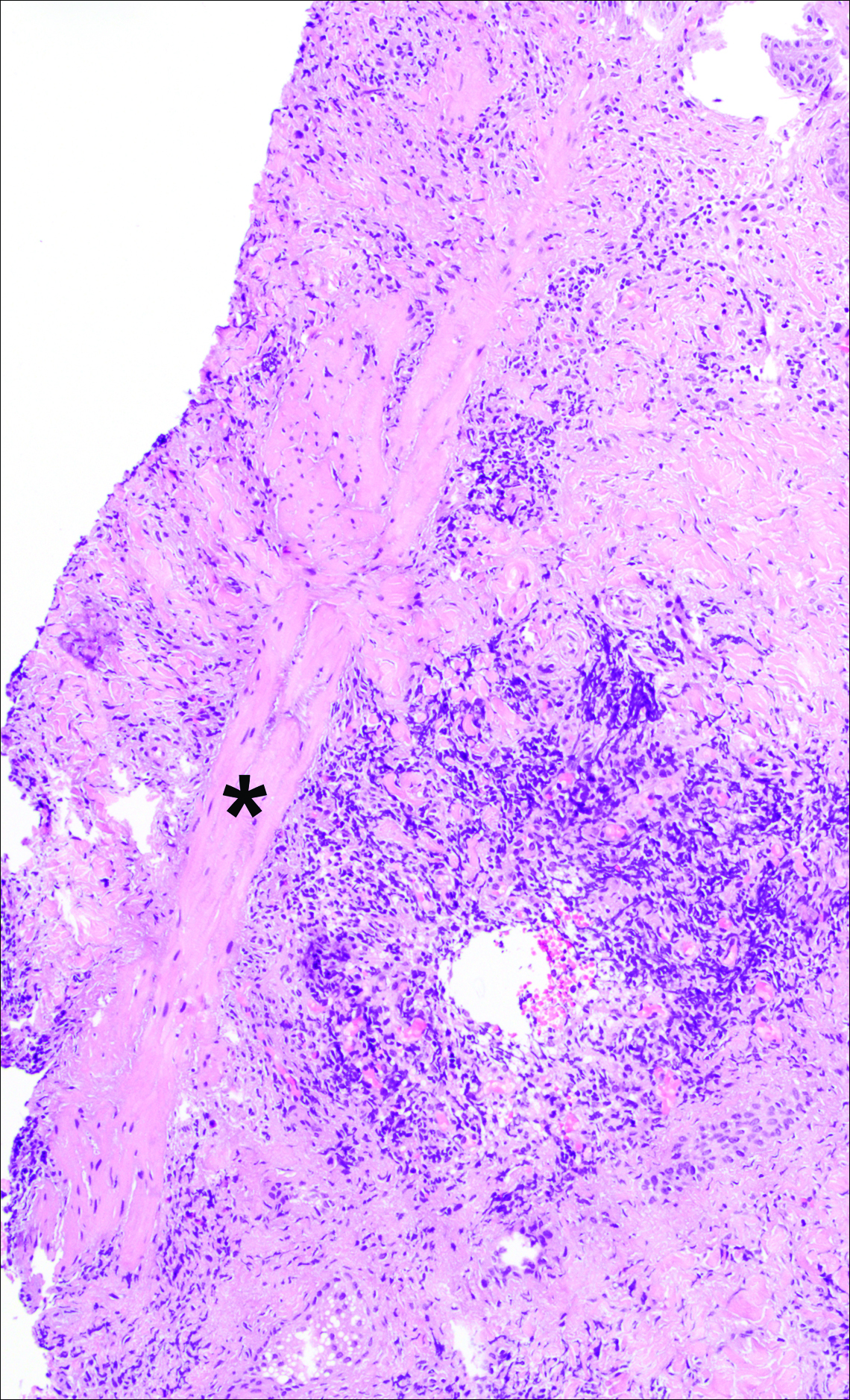
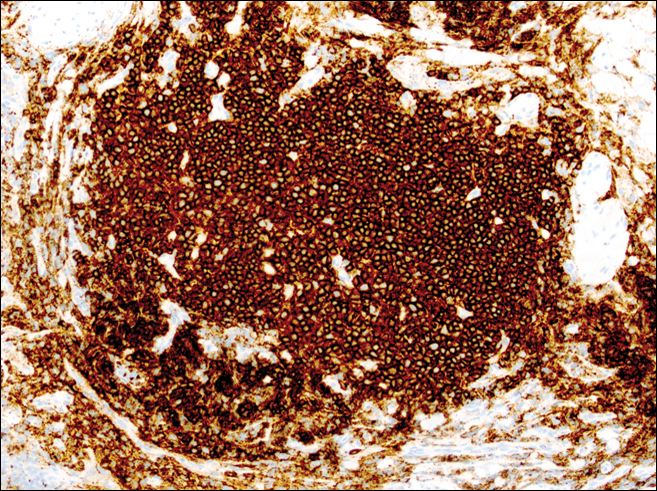
A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).
Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Practice Points
- Tick bite alopecia should be included in the differential diagnosis of both solitary and moth-eaten lesions of localized hair loss.
- In most cases, hair regrowth can be expected in a lesion of tick bite alopecia.