User login
Persistent Pruritic Papules on the Buttocks
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
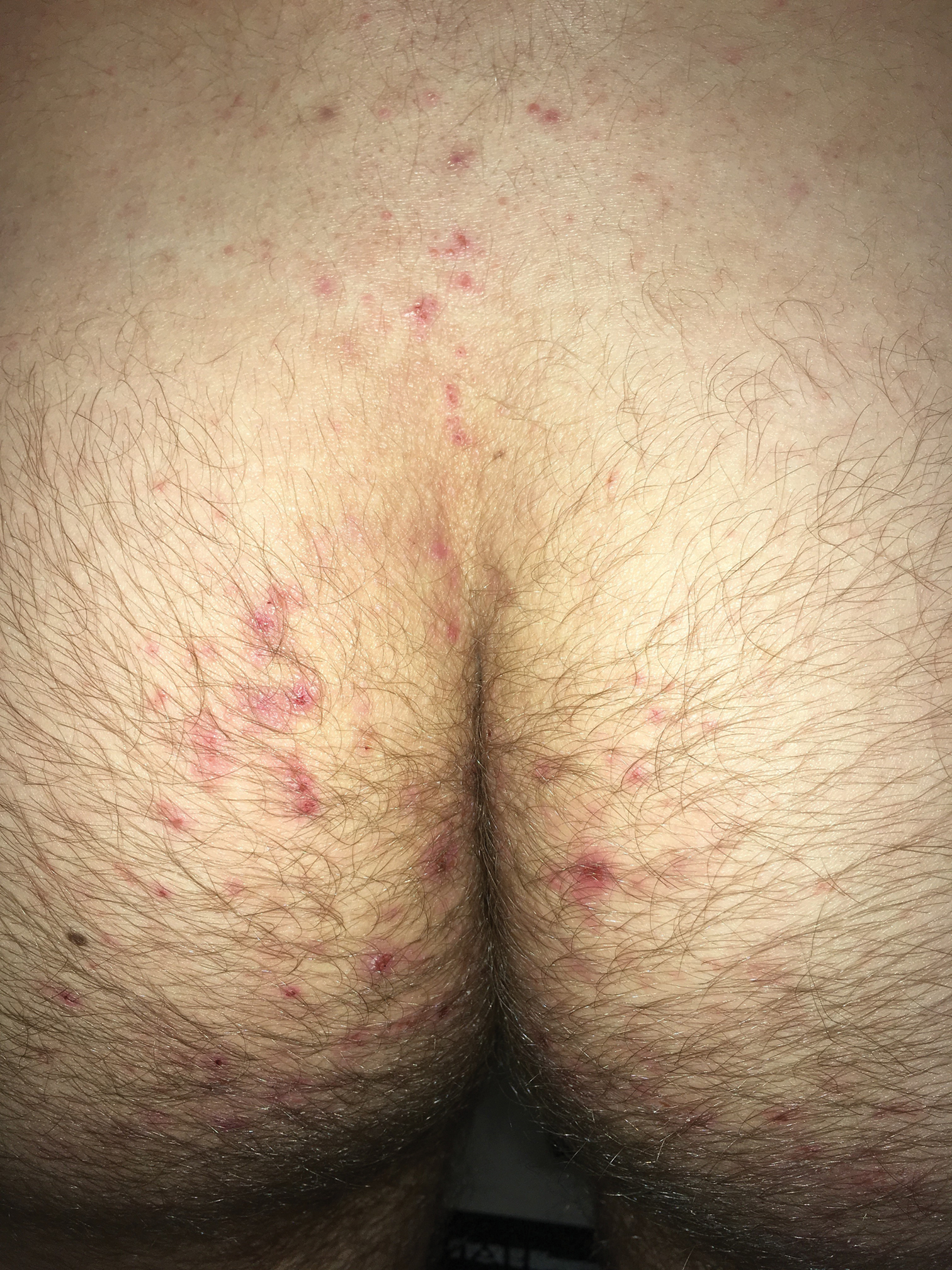
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3
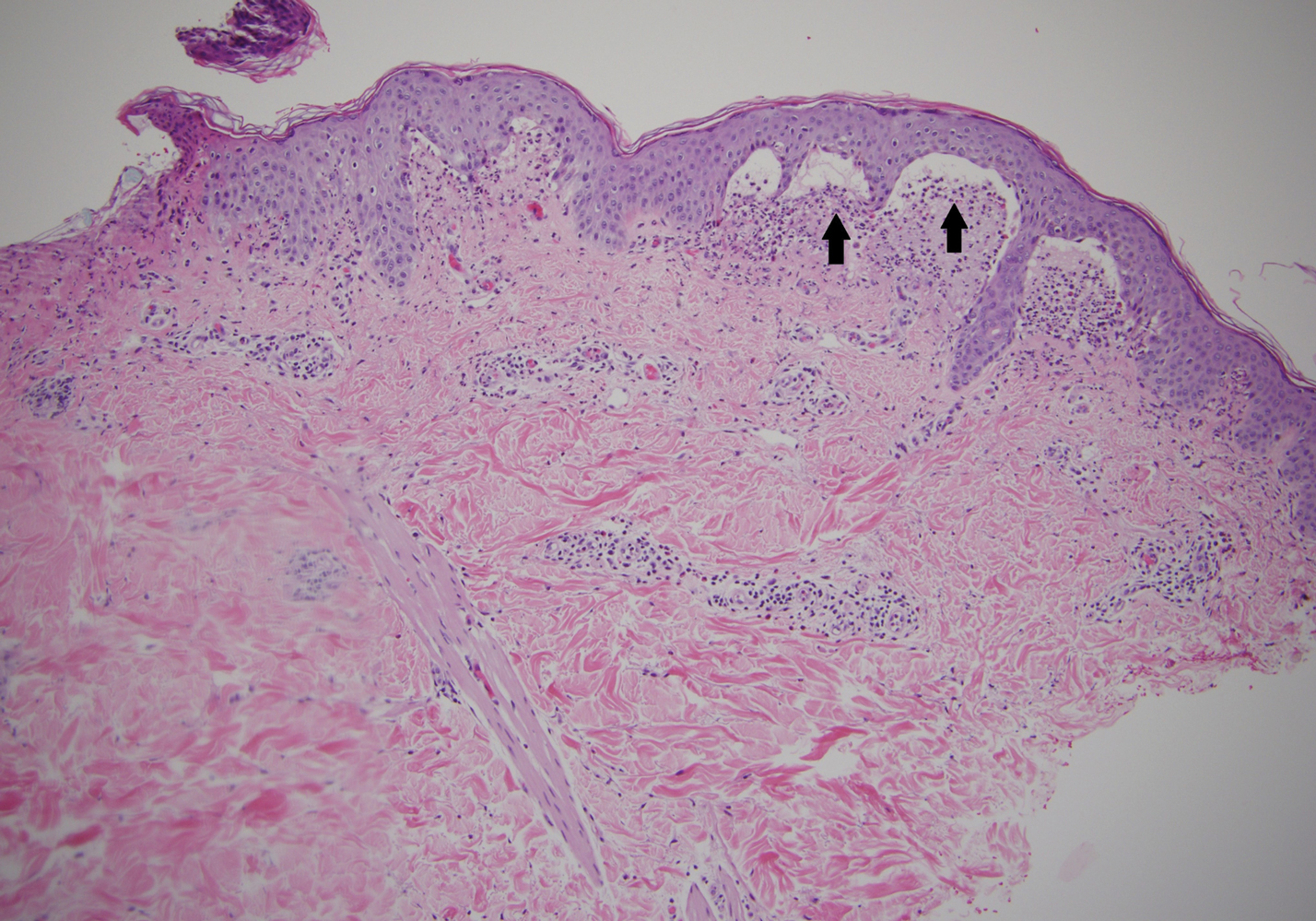
A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6
Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3
A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6
Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3
A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6
Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
A 19-year-old man presented to the dermatology clinic with intermittent pruritic lesions that began on the bilateral buttocks when he was living in Reserve Officers' Training Corps dormitories several months prior. The eruption then spread to involve the penis, suprapubic area, periumbilical area, and flanks. The patient attempted to treat the lesions with topical antifungals prior to evaluation in the emergency department where he was treated with permethrin 5% on 2 separate occasions without any improvement. A medical history was normal, and he denied recent travel, animal contacts, or new medications. Physical examination revealed several 2- to 4-mm erythematous papules and superficial erosions with an ill-defined erythematous background most notable on the penis, suprapubic area, periumbilical area, flanks, and buttocks.