User login
Things We Do for No Reason™: Emergent Hemodialysis After Intravascular Iodinated Contrast Exposure in Chronic Hemodialysis Patients
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
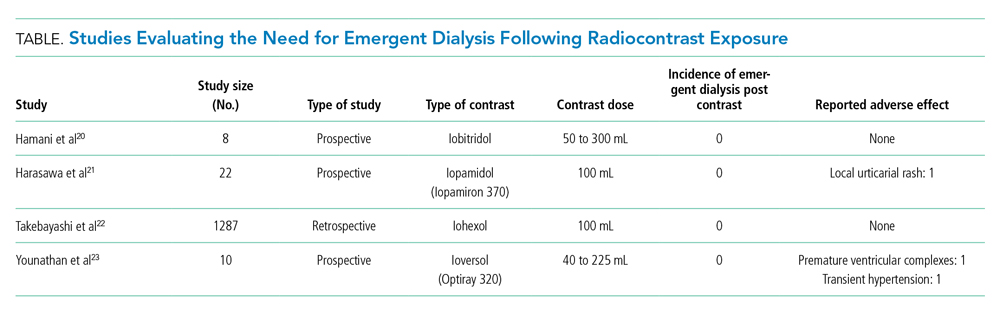
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™" (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 56-year-old anuric man with end-stage renal disease (ESRD) on maintenance hemodialysis (HD) for an acute coronary syndrome. He received his regularly scheduled HD the day before admission. Cardiology delays his coronary catheterization until nephrology can arrange for HD immediately after angiography. After angiography, the patient receives emergent HD even though he had acceptable metabolic parameters and did not show signs or symptoms of volume overload. The hospitalist wonders whether arranging emergent HD after the procedure with intravascular (IV) contrast was necessary for this patient.
BACKGROUND
Of the approximately 600 million radiological examinations performed annually, 75 million require iodinated contrast material (ICM).1 ICM are small, highly diffusible, minimally protein-bound molecules. They are not metabolized by humans, with healthy kidneys excreting approximately 99.8% of the administered dose within 24 hours.2 ICM has been associated with acute kidney injury (AKI), but its deleterious effects have not been thoroughly described, and the incidence and severity of contrast-associated nephropathy vary among studies.3 Not surprisingly, the strongest independent patient-related risk factor for developing contrast-induced AKI is preexisting chronic kidney disease.4 In patients with ESRD, the biliary system slowly clears the contrast, leading to long-standing retention. Newer low- or iso-osmolar contrast material is now used rather than older, conventional high-osmolality agents. These agents are less likely to lead to AKI.5
Recent studies have challenged the association between AKI and ICM administration.6-8 In 2015, the American College of Radiology endorsed the terms contrast-associated acute kidney injury and contrast-induced acute kidney injury, instead of the contrast-induced nephropathy, to avoid the uncertainty about the causal relationship between AKI and ICM.9 ESRD patients have little or no functional renal tissue and are on renal replacement therapy, either HD or peritoneal dialysis. However, physicians apprehensive about the renal and cardiovascular toxicity caused by retained ICM might request postprocedural HD to promote quicker contrast clearance in patients with ESRD.
WHY YOU MIGHT THINK PERFORMING EMERGENT HEMODIALYSIS AFTER IV CONTRAST IS NECESSARY
Clinicians divide patients with ESRD into two groups depending on their ability to produce urine. Those who produce urine have residual renal function (RRF), which independently predicts survival.10 Among a cohort of peritoneal and HD patients, Maiorca et al described a 40% reduction in the risk of death for each 1 mL/min increase in glomerular filtration rate (GFR).10 Therefore, patients on maintenance dialysis who have RRF are considered similar to patients with AKI and eGFR <30 mL/min/1.73 m2.9 Clinicians might worry that contrast retention could reduce RRF by inducing AKI.2,4,11
Volume overload is a second concern with ICM administration in ESRD patients. In mice, higher-osmolality ICM produced acute pulmonary edema, leading to death.12 A rapid bolus of diatrizoate caused transient intravascular expansion as reflected by an average decrease in hemoglobin of 0.5 to 0.8 g/dL, depending on the osmolality of the agent.12
Conventional high-osmolar ICM also depresses myocardial contractile force, sinoatrial automaticity, and atrioventricular nodal conduction, resulting in bradycardia, transient heart blocks, and increased risk of ventricular fibrillation.12 High-osmolar calcium-binding ICM transiently reduces systemic vascular resistance, resulting in transient hypotension and increased cardiac output. Researchers linked these adverse cardiac effects to the high-osmolality ionic ICM, not newer agents.12 In one study of adverse outcomes linked to ICM, 36% of patients with normal kidney function exposed to contrast developed an adverse reaction; 2% of patients developed level 4 (severe) adverse reactions.13 The study noted a significantly increased risk of bradycardia (relative risk [RR], 17.9), hypotension (RR, 6.3), and angina (RR, 3.4) among those who received high-osmolality contrast agents.
HD removes 72% to 82% of ICM at 4 hours.14 Armed with data from mice or small-population studies that demonstrated the toxic effects of conventional high-osmolar ICM, many radiologists and clinicians recommend post-contrast HD for patients at high risk for contrast-induced AKI and chronic HD patients.2 Moon et al suggested prophylactic HD for quicker removal of the iodinated contrast medium to prevent reduction in renal function among high-risk patients after angiographic interventions.15
WHY THERE IS LITTLE REASON TO HEMODIALYZE AFTER CONTRAST EXPOSURE
Over the last 3 decades, we have transitioned from conventional radiocontrast to low-osmolality agents that are not directly toxic to the kidneys. Iodixanol, iohexol, and iopromide exposure during intravascular radiological procedures did not result in a decline of RRF among well-hydrated peritoneal dialysis patients with RRF.16,17 The limited analysis of HD trials in the systematic review by Cruz et al concluded that periprocedural HD in patients with chronic kidney disease did not decrease the incidence of radiocontrast-associated nephropathy.18 A meta-analysis of nine studies (434 patients) concluded that ICM administration does not cause significant reduction of residual function in dialysis patients.19 Because anuric ESRD patients have no salvageable renal function and are on HD, managing AKI seems irrelevant.
Although volume overload is an important consideration, the theoretical increase in intravascular volume with administration of 100 mL of 1500 mOsm/L of conventional ICM to a 70 kg-patient is only 120 mL.14 More importantly, use of low-osmolar ICM substantially reduces any significant volume shifts.
Studies have not associated low-osmolality ICM with cardiovascular adverse effects.20-23 A retrospective study by Takebayashi et al showed an absence of serious adverse reactions to low-osmolar contrast media when HD was performed on their regular HD schedule.22 Older, smaller prospective trials did not show a need for periprocedural HD after ICM exposure.20,21,23 In a prospective study of 10 ESRD patients, Younathan et al assessed for postprocedural adverse effects of non-ionic contrast material and found that none required emergent HD.23 Similarly, Hamani et al and Harasawa et al did not observe hemodynamic and cardiopulmonary effects of IV contrast in chronic HD patients (Table).20,21 Injection of non-ionic contrast material in patients on chronic HD did not produce significant changes in blood pressure, electrocardiogram results, osmolality, extracellular fluid volume, or body weight.23 Finally, the vasoconstrictor-mediated ischemic injury of ICM occurs within minutes of administration, making dialysis performed hours later of little benefit.
HD is associated with adverse effects, including hypotension, which can jeopardize cardiovascular recovery after a myocardial infarction.24 The retrospective study performed by Fujimoto et al demonstrated dialytic complications in 24% of patients dialyzed the day of angiography.25 They noted that the amount of contrast agent administered independently predicted intradialytic hypotension.25,26
Delays in performing cardiac revascularizations are associated with an increase in 30-day mortality. The 30-day mortality rates of patients diagnosed with ST-elevation myocardial infarction who underwent revascularization in <60 minutes, 61 to 75 minutes, 76 to 90 minutes, and >90 minutes from study enrollment were 1%, 3.7%, 4%, and 6.7%, respectively.27 Delayed diagnosis of pulmonary embolism or acute limb ischemia was associated with increased rates of complications and mortality.28,29 The benefits of essential radiocontrast procedures outweigh the potential cardiovascular and cerebrovascular complications for HD patients. Considering the evidence, the American College of Radiology’s 2020 Manual on Contrast Media and the European Society for Urogenital Radiology’s 2018 guidelines on contrast medium administration in patients on HD concluded that an extra session or a change in the usual timing of HD is unnecessary.13,30
WHAT YOU SHOULD DO INSTEAD
HD performed post-contrast exposure does not provide any protective benefit, regardless of the degree of RRF (anuric ESRD or otherwise), making the timing of HD irrelevant. Do not delay studies that provide essential information for clinical management of high-risk conditions. The decision to perform HD in a patient who needs contrast-enhanced studies should be made independent of whether they will receive contrast.
RECOMMENDATIONS
- Immediate post-procedural HD after ICM exposure in ESRD patients is not required.
- Do not delay vital diagnostic or therapeutic procedures requiring ICM in ESRD patients.
- The indication for HD is independent of contrast exposure in ESRD patients.
CONCLUSION
The hospitalist did not need to arrange emergent post-procedural HD because it does not improve clinical outcomes. Delaying potentially lifesaving diagnostic and therapeutic measures involving the use of radiocontrast to secure post-radiocontrast HD could lead to worse outcomes.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
1. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005;209(2):185-187. https://doi.org/10.1016/j.tox.2004.12.020
2. Deray G. Dialysis and iodinated contrast media. Kidney Int Suppl. 2006(100):S25-29. https://doi.org/ 10.1038/sj.ki.5000371
3. American College of Radiology. ACR manual on contrast media. Published 2020. Accessed July 18, 2021. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf
4. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-2155. https://doi.org/10.1056/NEJMra1805256
5. Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. 2020;75(1):105-113. https://doi.org/10.1053/j.ajkd.2019.05.022
6. Ehrmann S, Aronson D, Hinson JS. Contrast-associated acute kidney injury is a myth: yes. Intensive Care Med. 2018;44(1):104-106. https://doi.org/10.1007/s00134-017-4950-6
7. Kashani K, Levin A, Schetz M. Contrast-associated acute kidney injury is a myth: we are not sure. Intensive Care Med. 2018;44(1):110-114. https://doi.org/10.1007/s00134-017-4970-2
8. Weisbord SD, du Cheryon D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. 2018;44(1):107-109. https://doi.org/10.1007/s00134-017-5015-6
9. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. https://doi.org/10.1148/radiol.2019192094
10. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53(6):1068-1081. https://doi.org/10.1053/j.ajkd.2009.02.012
11. Hsieh MS, Chiu CS, How CK, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2016;95(16):e3388. https://doi.org/10.1097/MD.0000000000003388
12. Hirshfeld JW, Jr. Cardiovascular effects of iodinate contrast agents. Am J Cardiol. 1990;66(14):9F-17F. https://doi.org/10.1016/0002-9149(90)90635-e
13. Steinberg EP, Moore RD, Powe NR, et al. Safety and cost effectiveness of high-osmolality as compared with low-osmolality contrast material in patients undergoing cardiac angiography. N Engl J Med. 1992;326(7):425-430. https://doi.org/10.1056/NEJM199202133260701
14. Rodby RA. Preventing complications of radiographic contrast media: Is there a role for dialysis? Sem Dial. 2007;20(1):19-23. https://doi.org/10.1111/j.1525-139X.2007.00233.x
15. Moon SS, Bäck SE, Kurkus J, Nilsson-Ehle P. Hemodialysis for elimination of the nonionic contrast medium iohexol after angiography in patients with impaired renal function. Nephron. 1995;70(4):430-437. https://doi.org/10.1159/000188641
16. Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21(5):1334-1339. https://doi.org/10.1093/ndt/gfi023
17. Moranne O, Willoteaux S, Pagniez D, Dequiedt P, Boulanger E. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21(4):1040-1045. https://doi.org/10.1093/ndt/gfi327
18. Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48(3):361-371. https://doi.org/10.1053/j.ajkd.2006.05.023
19. Oloko A, Talreja H, Davis A, et al. Does iodinated contrast affect residual renal function in dialysis patients? a systematic review and meta-analysis. Nephron. 2020;144(4):176-184. https://doi.org/10.1159/000505576
20. Hamani A, Petitclerc T, Jacobs C, Deray G. Is dialysis indicated immediately after administration of iodinated contrast agents in patients on haemodialysis? Nephrol Dial Transplant. 1998;13:1051-1052.
21. Harasawa H, Yamazaki C, Masuko K. Side effects and pharmacokinetics of nonionic iodinated contrast medium in hemodialized patients. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50(12):1524-1531.
22. Takebayashi S, Hidai H, Chiba T. No need for immediate dialysis after administration of low-osmolarity contrast medium in patients undergoing hemodialysis. Am J Kidney Dis. 2000;36(1):226. https://doi.org/10.1053/ajkd.2000.8301
23. Younathan CM, Kaude JV, Cook MD, Shaw GS, Peterson JC. Dialysis not indicated immediately after administration of nonionic contrast agents in patients with end-stage renal disease treated by maintenance dialysis. AJR. Am J Roentgenol. 1994;163:969-971. https://doi.org/10.2214/ajr.163.4.8092045
24. Coritsidis G, Sutariya D, Stern A, et al. Does timing of dialysis in patients with ESRD and acute myocardial infarcts affect morbidity or mortality? Clin J Am Soc Nephrol. 2009;4(8):1324-1330. https://doi.org/10.2215/CJN.04470908
25. Fujimoto M, Ishikawa E, Haruki A, et al. Hemodialysis complications after angiography and its risk factors. Nihon Toseki Igakkai Zasshi. 2015;48(5):269-274. https://doi.org/10.4009/jsdt.48.269
26. Tachibana K, Kida H, Uenoyama M, Nakamura T, Yamada T, Hayahi T. Risk factors for intradialytic hypotension after percutaneous coronary interventions. Nihon Toseki Igakkai Zasshi. 2019;52(4):227-232. https://doi.org/10.4009/jsdt.52.227
27. Berger PB, Ellis SG, Holmes DR Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Circulation. 1999;100(1):14-20. https://doi.org/10.1161/01.cir.100.1.14
28. Nagasheth K, Nassiri N, Shafritz R, Rahimi S. Delayed revascularization for acute lower extremity ischemia leads to increased mortality. J Vasc Surg. 2016;63(6S):121S-122S.
29. Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA Jr. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592-598. https://doi.org/10.1197/j.aem.2007.03.1356
30. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Accessed July 20, 2021. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
© 2021 Society of Hospital Medicine