User login
Group Clinic for Chemoprevention of Squamous Cell Carcinoma: A Pilot Study
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
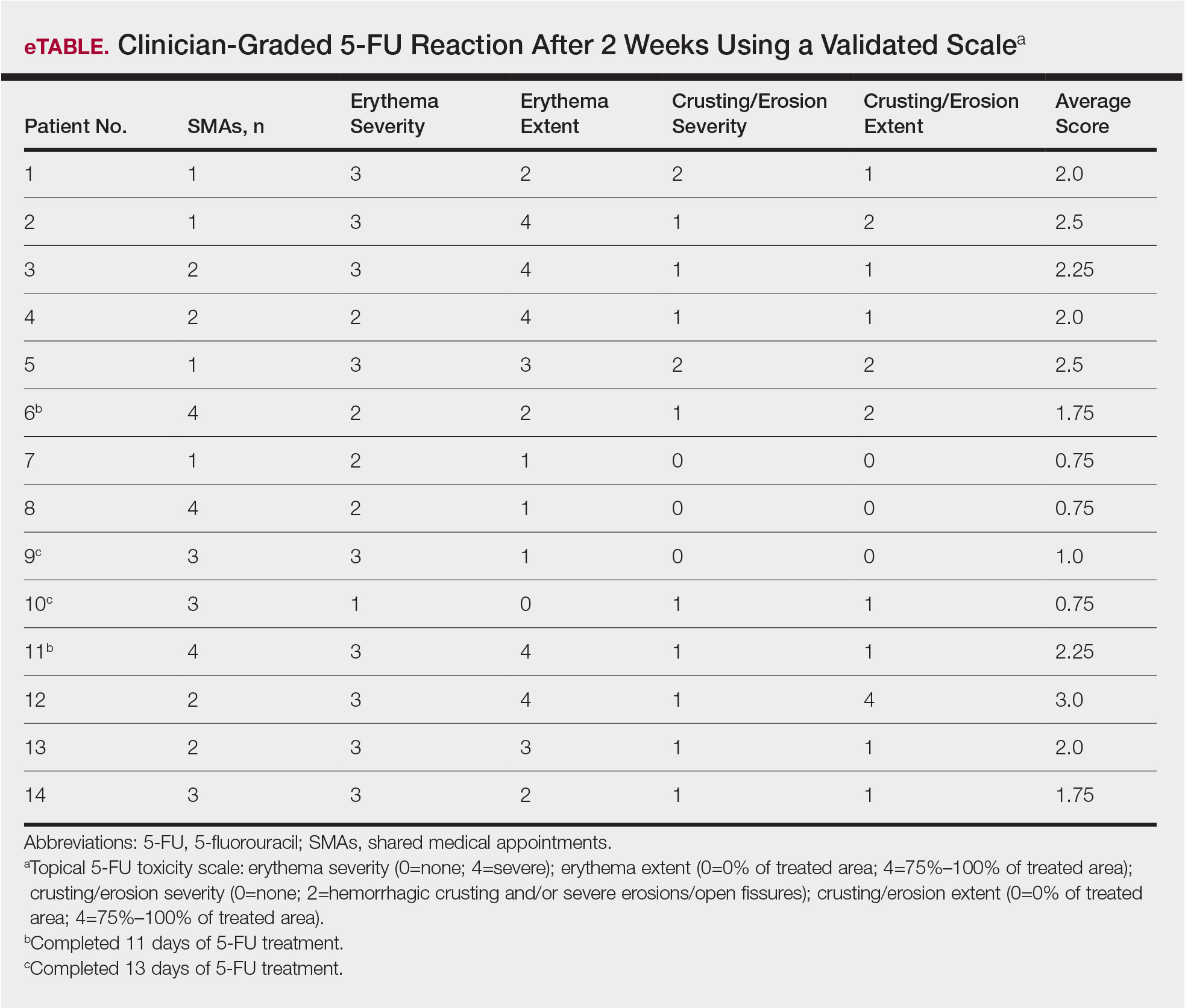
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Practice Points
- Shared medical appointments (SMAs) enhance patient experience with topical 5-fluorouracil (5-FU) treatment of actinic keratosis (AK).
- Dermatologists should consider utilizing the SMA model for their patients being treated with 5-FU, as patients demonstrated a positive emotional response to 5-FU therapy in the group clinic setting.