User login
The Challenges of Normal Pressure Hydrocephalus: A Case-Based Review
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
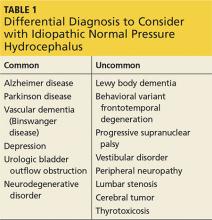
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
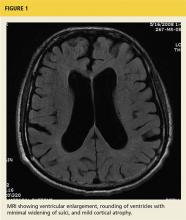
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
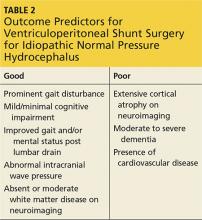
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.