User login
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
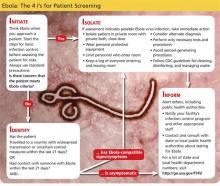
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
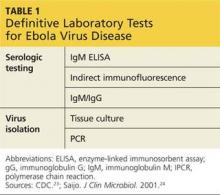
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
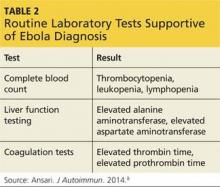
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
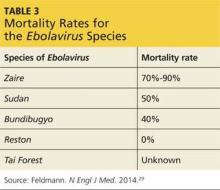
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.