User login
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
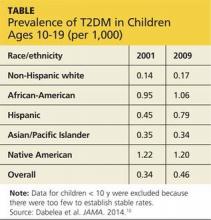
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.