User login
Gadolinium Deposition Disease: A Case Report and the Prevalence of Enhanced MRI Procedures Within the Veterans Health Administration
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
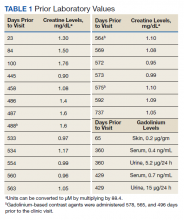
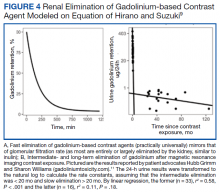
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
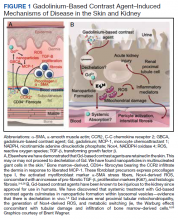
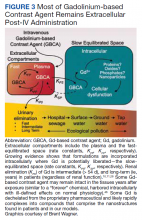
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
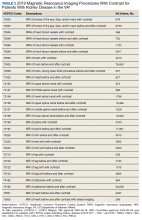
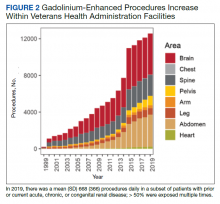
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
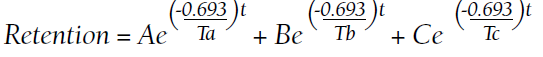
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
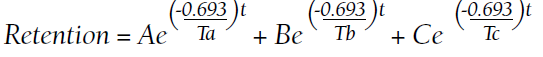
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
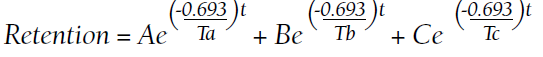
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
Rosuvastatin-Induced Rhabdomyolysis, Pancreatitis, Transaminitis, and Acute Kidney Injury
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Changing medications within a drug class requires considering the indication and dosage, possible adverse effects, and drug-drug interactions.
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
Attention should be paid to changing a tolerated medication to another within its class. Many drugs approved by the US Food and Drug Administration (FDA), have equivalent therapeutic properties as existing drugs. Rarely do such medications share the same potency and adverse effect (AE) profile.
Case Presentation
A 77-year-old man presented to the emergency department (ED) at the Raymond G. Murphy Medical Center in Albuquerque, New Mexico, with a 1-month history of progressive muscle weakness, which was so severe that he required assistance rising from chairs. The symptoms began when he switched from atorvastatin 40 mg daily to rosuvastatin 40 mg daily. A nephrology consultation was requested for an elevated plasma creatinine.
The patient reported strict adherence to his prescribed medications. In the days following the switch to rosuvastatin, he noticed that his urine turned black. He described the color as “like burnt coffee.” The color gradually cleared before his ED presentation. The patient stopped taking rosuvastatin the day prior to presentation and noted improvement of his symptoms. Review of symptoms was significant for lower extremity paresthesia and numbness the day he started rosuvastatin. He had no symptoms of decompensated heart failure and no recent exacerbations requiring alteration of his diuretic regimen.
The patient’s medical history was significant for traumatic brain injury with complex partial seizures, carpal tunnel syndrome, dyslipidemia, coronary artery disease with percutaneous intervention to the right coronary artery in the late 1990s, atrial fibrillation and ventricular tachycardia, status post implantable cardioverter defibrillator, heart failure with reduced ejection fraction (25%) attributed to ischemic cardiomyopathy, hypertension, lower urinary tract symptoms/prostatism, and previous bladder cancer. In the mid-1960s, the patient served in the US Army and had been deployed to South Korea. After the service, he worked for the local city government. He was retired for about 15 years. He reported no tobacco, alcohol, or recreational drug use and no tattoos. He did not require prior blood or blood product transfusions. None of his family members—parents, siblings, or children—had any history of kidney disease.
The patient’s outpatient medications included levetiracetam 750 mg twice daily, melatonin 9 mg at night, menthol 16%/methyl-salicylate 30% topically up to 4 times per day as needed, aspirin 81 mg once daily, fish oil 1000 mg twice daily, amiodarone 400 mg twice daily, hydralazine 20 mg 3 times daily, isosorbide mononitrate 60 mg daily, metoprolol succinate 100 mg daily, and tamsulosin 0.4 mg at night. His vital signs were stable: afebrile (97.5 ºF), normocardic (74 beats per minute), normotensive (118/78 mm Hg), and normoxic (98% on room air). On examination, he appeared elderly, somewhat frail, and chronically ill but in no acute distress. Affect was pleasant and appropriate, attention was high, and his thought process was logical. He had sparse, grey scalp hair. Extraocular movements were intact. Oral mucosa was pink and moist. His back was nontender, and there was no costovertebral tenderness bilaterally. The patient was in no respiratory distress, with a slightly hyperresonant chest to percussion bilaterally, very faint inspiratory basilar crepitant rales (that cleared with repeat inspiration), and was otherwise clear to auscultation throughout. An outline of an implanted pacemaker was evident on the chest under his left clavicle, with a laterally displaced apical impulse. The rate was normal and the rhythm was regular. Upper extremities demonstrated papyraceous skin but without cyanosis, clubbing, or edema. Radial pulses were slightly diminished. He had no lower extremity edema.
His laboratory values are provided in Table 1. Kidney function was stable months prior to admission. Of note, the blood urea nitrogen and plasma creatinine were increased from his baseline up to 47 and 5.89 mg/dL, respectively. The serum glutamic-oxaloacetic transaminase and serum glutamic pyruvic transaminase were 1051 U/L and 408 U/L, respectively. Plasma amylase and lipase levels also were elevated, 230 U/L and 892 U/L, respectively. Creatine kinase was 41,099 U/L. Urinalysis demonstrated a specific gravity of 1.017, pH of 5, and a large amount of blood (92 red blood cells/high power field).
A 12-lead electrocardiogram demonstrated a sinus rhythm, PR interval of 0.20 ms, narrow QRS with a leftward frontal axis deviation, R-transition between precordial leads V1 and V2, and flattening of the ST segments in III, V1-V3 (Figure 1). A portable chest X-ray demonstrated clear lung fields, no evidence of effusion in the costophrenic area. Ultrasonography was conducted at the time of the examination (Figure 2). The kidneys were smoothly contoured, each measuring > 10 cm; there was an exophytic cyst on the left. Otherwise, the cortices, perhaps slightly echogenic, did not appear diminished. The bladder was not abnormally enlarged.
Rosuvastatin-induced rhabdomyolysis, pancreatitis, transaminitis, and drug-induced acute kidney injury were considered high among the diagnostic differentials. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitor was stopped, and he was prescribed an acute renal insufficiency diet. All laboratory parameters improved with this change (Figure 3). Two months after presentation (and with rosuvastatin added to his list of adverse reactions), all symptoms resolved and his plasma creatinine reached a nadir of 1.22 mg/dL.
Discussion
Statin-class drugs inhibit the HMG-CoA reductase (Table 2). Upregulation of low-density lipoprotein cholesterol (LDL-C) receptors in the liver result in increased LDL-C uptake and cholesterol catabolism.1 Prescribed inhibitors of the HMG-CoA reductase—statins—are known to reduce mortality due to cardiovascular disease (CVD). Much like any other pharmaceutical agent with any measurable potency, HMG-CoA inhibitors can have AEs. Statin therapy has been associated with pancreatitis.2 Muscle toxicity is a complication of HMG-CoA reductase inhibitors, and statin-associated symptoms are a leading cause of nonadherence.3 Rosuvastatin had higher AE and drug reactions compared with that of atorvastatin and pitavastatin (35.6%, 8.7%, and 22.2%, respectively) in clinical trials for approval.4 We have reported concomitant adermatopathic dermatomyositis with statin-induced myopathy in a 48-year-old man from simvastatin (40 to 80 mg daily).1
Toxin-induced myopathy should be considered early in the differential diagnosis of weakness.5 All HMG-CoA inhibitors have been associated with acute kidney injury, particularly at high doses and also are known to induce myopathies, sometimes with inclusion bodies.1 Muscle-related AEs correlate with the potency of an HMG-CoA reductase inhibitor according to an analysis using the FDA AE Reporting System (AERS).6 Myalgia and rhabdomyolysis are well-known AEs of this class of medications. Furthermore, type II muscle atrophy—particularly in the proximal limb muscles—has been reported.5 Patients may have difficulty rising from chairs.1 Rosuvastatin had the strongest signal for muscular AEs (eg, myalgia, rhabdomyolysis, increased creatine phosphokinase level) from an FDA analysis of AERS.7
Rosuvastatin is the only HMG-CoA reductase inhibitor that causes dose-dependent increases in proteinuria and hematuria (Figure 4).8 Rosuvastatin at a 5-mg dose may induce 4 times the proteinuria as a placebo. Typically, other statins potentially reduce proteinuria (without hematuria). Proteinuria may be induced by rosuvastatin even at low doses.8 Proteinuria is attributed to how rosuvastatin impacts proximal tubular function.9 The drug is transported into the proximal tubule by the organic anion transporter-3. Acute kidney injury has been associated with several statins, including rosuvastatin.7,10 This may be associated with denuded tubular epithelia, active urinary sediment, acute tubular toxicity, vacuolated epithelial cells, and tubular cell casts. Unlike atorvastatin, the increase in proteinuria and hematuria also is dose dependent.
In patients with renal insufficiency (short of end-stage renal disease [ESRD]), most statins other than rosuvastatin are well tolerated and recommended for reduction of overall and CVD mortality risk. However, these benefits seem to diminish once ESRD is reached. Atorvastatin did not impact CVD mortality in patients with type 2 diabetes mellitus (T2DM) and ESRD (despite decreasing LDL-C).11 The AURORA study randomized 10 mg of statin vs placebo in 2776 maintenance dialysis patients aged 50 to 80 years. Rosuvastatin lowered the LDL-C but did not affect all-cause mortality (13.5 vs 14.0 events per 100 patient-years). Patients randomized to rosuvastatin had more than twice as many unclassified strokes (9 vs 4). Rosuvastatin, although efficacious in reducing LDL-C, had no impact on CVD mortality, nonfatal myocardial infarction, or nonfatal stroke.12 Post hoc analysis demonstrated that in patients with T2DM with ESRD the hazard ratio for hemorrhagic stroke was 5.2.13
Rosuvastatin ranked lower than lovastatin, pravastatin, simvastatin, atorvastatin, and fluvastatin with respect to reduction of all-cause mortality in trials of participants with or without prior coronary artery disease.14 AEs, such as rhabdomyolysis, proteinuria, nephropathy, renal failure, liver, and muscle toxicity are higher with rosuvastatin than other medications in its class.15
Conclusions
For patients with existing CVD, standard clinical practice is to encourage increased and regular physical activity, cholesterol-lowering diets, weight loss, and smoking cessation. Hypertension should be treated. Glycemia should be well controlled in the setting of T2DM. β-blockers may be beneficial in those with histories of myocardial infarction or heart failure with reduced systolic function. Statins are a valuable tool in the treatment of dyslipidemia.
Statin-induced muscle symptoms are a major reason for discontinuation and nonadherence.16 Statin-induced myalgia, myositis, and myopathy have been used interchangeably.17 Rhabdomyolysis, myalgia, increased creatine kinase, statin myopathy, and immune-mediated necrotizing myopathy are among the clinical phenotypes caused by statins.17 There are 33,695 serious cases—1808 deaths—reported with rosuvastatin in the FDA AERS as of June 30, 2021. Myalgia, pain in extremity, muscle spasms, pain, and arthralgia top the list of AEs. When statin-induced symptoms occur, adherence is rarely improved by dismissive clinicians.18
Drugs in the same class often have common therapeutic properties. Potencies and AE profiles are seldom uniform. The decision to add or change the brand of medication within a class should be balanced with considerations for the indication, duplications, simplification, AEs, appropriate dosage, and drug-drug interactions.
Acknowledgments
Brent Wagner is funded by a US Department of Veterans Affairs Merit Award (I01 BX001958), a National Institutes of Health R01 grant (DK-102085), Dialysis Clinic, Inc., and partially supported by the University of New Mexico Brain and Behavioral Health Institute (BBHI 2018-1008, 2020-21-002) and in part by the University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD); and the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust). Brent Wagner is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism Center of Biomedical Research Excellence (AIM CoBRE) supported by NIH grant P20GM121176.
Funding
National Institutes of Health Grant R01 DK-102085, Dialysis Clinic Inc., VA Merit Award I01 BX001958, Center for Integrated Nanotechnologies User Agreement 2019AU0120, Brain & Behavioral Health Institute (grants 2018-1008, 2020-21-002), University of New Mexico’s Signature Program in Cardiovascular and Metabolic Disease (CVMD), the University of New Mexico School of Medicine Research Allocation Committee (C-2459-RAC, New Mexico Medical Trust) and a metabolomics voucher from the AIM Center (NIH P20GM121176).
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0
1. Wagner B, Kagan-Hallet KS, Russell IJ. Concomitant presentation of adermatopathic dermatomyositis, statin myopathy, fibromyalgia syndrome, piriformis muscle myofascial pain and diabetic neuropathy. J Musculoskeletal Pain. 2003;11(2):25-30. doi:10.1300/J094v11n02_05
2. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy [published correction appears in Lancet. 2017 Feb 11;389(10069):602]. Lancet. 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
3. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
4. Saku K, Zhang B, Noda K; PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011;75(6):1493-1505. doi:10.1253/circj.cj-10-1281
5. Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18(3):695-718. doi:10.1016/s0733-8619(05)70219-x
6. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866. doi:10.1371/journal.pone.0042866
7. Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi:10.1371/journal.pone.0028124
8. Tiwari A. An overview of statin-associated proteinuria. Drug Discov Today. 2006;11(9-10):458-464. doi:10.1016/j.drudis.2006.03.017
9. Verhulst A, Sayer R, De Broe ME, D’Haese PC, Brown CD. Human proximal tubular epithelium actively secretes but does not retain rosuvastatin. Mol Pharmacol. 2008;74(4):1084-1091. doi:10.1124/mol.108.047647
10. Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152-160. doi:10.1016/s0002-9149(03)00530-7
11. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis [published correction appears in N Engl J Med. 2005 Oct 13;353(15):1640]. N Engl J Med. 2005;353(3):238-248. doi:10.1056/NEJMoa043545
12. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis [published correction appears in N Engl J Med. 2010 Apr 15;362(15):1450]. N Engl J Med. 2009;360(14):1395-1407. doi:10.1056/NEJMoa0810177
13. Holdaas H, Holme I, Schmieder RE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335-1341. doi:10.1681/ASN.2010090987
14. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20(4):641-657. doi:10.1177/2047487313480435
15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111(23):3051-3057. doi:10.1161/CIRCULATIONAHA.105.555482
16. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328-350. doi:10.1161/CIRCRESAHA.118.312782
17. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215-224. doi:10.1080/1744666X.2018.1440206
18. Koslik HJ, Meskimen AH, Golomb BA. Physicians’ Experiences as patients with statin side effects: a case series. Drug Saf Case Rep. 2017;4(1):3. doi:10.1007/s40800-017-0045-0