User login
Manage Your Dermatology Practice: Treating the More “Informed” Patient
The Internet has provided patients with a variety of resources to keep them informed; however, it is important for the dermatologist to dispel any misinformation. Dr. Gary Goldenberg discusses patients who self-diagnose and side effects of medications. The dermatologist is in the position to be able to empower the patient to use information online in a productive way.
The Internet has provided patients with a variety of resources to keep them informed; however, it is important for the dermatologist to dispel any misinformation. Dr. Gary Goldenberg discusses patients who self-diagnose and side effects of medications. The dermatologist is in the position to be able to empower the patient to use information online in a productive way.
The Internet has provided patients with a variety of resources to keep them informed; however, it is important for the dermatologist to dispel any misinformation. Dr. Gary Goldenberg discusses patients who self-diagnose and side effects of medications. The dermatologist is in the position to be able to empower the patient to use information online in a productive way.
New Systemic Therapies for Psoriasis
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
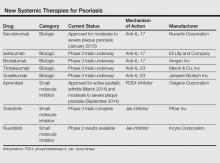
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
Psoriasis is a common chronic inflammatory skin disease affecting 1% to 8% of the world population, depending on the country.1 Psoriasis can greatly impact quality of life in affected individuals, even in those with limited body surface involvement.2 Studies have demonstrated a high degree of psychological distress associated with psoriasis, leading to depression and poor self-esteem.3
Over the last decade, our improved understanding of the autoimmune inflammatory pathways and the associated changing concepts in psoriasis pathogenesis have led to the development of biological drugs targeting specific components of effector immune mechanisms, and these biological drugs have revolutionized the treatment of psoriasis.4 Although response rates of these biological agents are greater compared to those of conventional systemic drugs,5 current biological drugs fail to demonstrate efficacy in some patients or lose their efficacy over time. In addition to the high costs associated with these drugs, these limitations have driven a continued search for alternative therapies.
Helper T cells (TH17) and the proinflammatory cytokine IL-17 have been shown to play a key role in the pathophysiology of psoriasis, bridging innate and adaptive immune responses. IL-17 is involved in the modulation of proinflammatory cytokines, hematopoietic growth factors, antimicrobial peptides, and chemokines. Increased TH17 activity and high levels of IL-17 have been found in psoriatic plaques, and increased levels of TH17 are found in the plasma of psoriasis patients.6 Increased IL-17 induces neutrophilia, inflammation, and angiogenesis.7 Other cytokines that are highly upregulated in involved skin are tumor necrosis factor a (TNF-α), IL-23, IL-22, and IL-21.8 IL-23 is involved in regulating TH17 cells and is a potent activator of keratinocyte proliferation.9 Blockade of IL-12/23 causes downregulation of TH17 and TH22 cell responses.10 As IL-17 has a key role in protecting skin and mucous membranes from bacterial and fungal infections, IL-17 inhibition can potentially interfere with the inflammatory cascade. However, available data suggest that sufficient residual IL-17 activity remains to maintain immunity against infections.11
Currently approved biological agents for psoriasis target proinflammatory cytokines such as TNF-α, or the p40 subunit of IL-12 and IL-23. A number of novel targeted therapies including biologics as well as small molecule inhibitors targeting various cytokines and molecules involved in the pathogenesis of psoriasis are currently in different stages of development (Table). These drugs include 3 IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); 2 IL-23 blockers (tildrakizumab and guselkumab); and small molecule inhibitors that target the kinase pathway including apremilast (a phosphodiesterase 4 [PDE4] inhibitor), as well as tofacitinib, baricitinib, and ruxolitinib (Janus kinase [Jak] inhibitors). Small molecule inhibitors can be administered orally and are less expensive to produce than biological agents. This article reviews available data on these new systemic agents in the pipeline.
Novel Biologics
Secukinumab
Secukinumab is a fully human monoclonal IgG1k antibody that selectively binds and neutralizes IL-17A.12 It is the first of the IL-17 antibodies to receive approval for the treatment of moderate to severe psoriasis. In 2 phase 3, double-blind, 52-week trials—ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) and FIXTURE (Full Year Investigative Examination of Secukinumab vs Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis)—participants were randomly assigned to receive subcutaneous secukinumab at doses of 300 mg (n=245 and n=327, respectively) or 150 mg (n=245 and n=327, respectively) once weekly for 5 weeks then every 4 weeks, or placebo (n=248 and n=326, respectively); in the FIXTURE study only, an etanercept group (n=326) was given a 50-mg dose twice weekly for 12 weeks then once weekly.13
In the ERASURE study, the proportion of participants showing a reduction of 75% or more in psoriasis area and severity index (PASI) score from baseline to week 12 was 81.6% with secukinumab 300 mg, 71.6% with secukinumab 150 mg, and 4.5% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 59.2% with secukinumab 300 mg and 39.1% with secukinumab 150 mg, which were both superior to placebo (1.2%). The proportion of participants who met the criteria for 100% reduction in PASI score at week 12 also was greater with each secukinumab dose than with placebo.13
In the FIXTURE study, the proportion of participants showing a reduction of 75% or more from baseline in PASI score at week 12 was 77.1% with secukinumab 300 mg, 67.0% with secukinumab 150 mg, 44.0% with etanercept, and 4.9% with placebo.13 Secondary end point results demonstrated the proportion of participants showing a 90% reduction in PASI score was 54.2% with secukinumab 300 mg, 41.9% with secukinumab 150 mg, 20.7% with etanercept, and 1.5% with placebo. The speed of response, which was assessed as the median time to a 50% reduction in mean PASI score from baseline, was significantly shorter with both doses of secukinumab (3.0 weeks and 3.9 weeks, respectively) than with etanercept (7.0 weeks)(P<.001 for both).13
In the FIXTURE study, incidences of adverse events (AEs) were similar in the secukinumab and etanercept groups during both the induction period and the entire treatment period.13 The most common AEs in the secukinumab groups were nasopharyngitis, headache, and diarrhea. The rates of infections or infestations during the induction period were 26.7% with secukinumab 300 mg, 30.9% with secukinumab 150 mg, 24.5% with etanercept, and 19.3% with placebo. Candidal infections were more common with secukinumab than with etanercept during the entire treatment period (4.7% and 2.3% of participants in the secukinumab 300 mg and 150 mg groups, respectively, reported mild or moderate candidal infections). None of these infections resulted in chronic mucocutaneous candidiasis or discontinuation of the study drug and all resolved on their own or with standard therapy. Candidal infection was reported in 1.2% of participants in the etanercept group. Responses at week 12 were sustained in the majority of participants through week 52 with continued secukinumab therapy every 4 weeks. Grade 3 neutropenia occurred in 1.0% of secukinumab-treated participants and in none of the participants in the etanercept group. There were no apparent dose-related differences between the secukinumab groups with respect to AEs, with the exception of mild and moderate candidal infections.13
These efficacy data are impressive and no specific serious safety concerns have been identified to date. However, IL-17A plays a key role in host defense, particularly in mucocutaneous immunity against Candida albicans,14 as well as in hematopoiesis through stimulation of granulopoiesis and neutrophil trafficking,15 and thus we need to remain watchful with regards to Candida albicans infections and neutropenia.
Ixekizumab
Ixekizumab is a humanized IgG4 anti–IL-17A monoclonal antibody. In a phase 2, double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomly assigned to receive 150-mg (n=28), 75-mg (n=29), 25-mg (n=30), or 10-mg (n=28) subcutaneous injections of ixekizumab or placebo (n=27) at weeks 0, 2, 4, 8, 12, and 16.16 At 12 weeks, the percentage of participants who achieved a 75% reduction in PASI score from baseline was significantly greater with ixekizumab (82.1% with 150-mg dose, 82.8% with 75-mg dose, 76.7% with 25-mg dose) than with placebo (7.7%)(P<.001 for each comparison), except with the 10-mg dose. Similarly, a greater percentage of participants in the same ixekizumab groups achieved a 90% reduction (71.4% with 150-mg dose, 58.6% with 75-mg dose, 50.0% with 25-mg dose) and a 100% reduction (39.3% with 150-mg dose, 37.9% with 75-mg dose) in PASI score compared to placebo (0%)(P<.001 for each comparison). Significant reductions in PASI scores were evident as early as week 1 in the 150-mg and 75-mg groups, and these reductions were sustained for 20 weeks (P<.05).16 Phase 3 studies of ixekizumab currently are underway.
Brodalumab
The third IL-17 blocker in the pipeline is brodalumab, a human monoclonal antibody against IL-17RA, which blocks signaling of IL-17A and IL-17F as well as the IL-17A/F heterodimer, all of which are involved in the inflammatory process of psoriasis. Brodalumab was evaluated in a phase 2, double-blind, placebo-controlled, dose-ranging study of 198 participants who were randomized to receive 70 mg (n=39), 140 mg (n=39), 210 mg (n=40), or 280 mg (n=42) of brodalumab or placebo (n=38).17 At week 12, improvements of at least 75% and at least 90% in PASI score were achieved by 77% and 72%, respectively, in the 140-mg group, and 82% and 75%, respectively, in the 210-mg group compared to 0% of the placebo group (P<.001 for all comparisons). One hundred percent improvement in PASI was achieved by 38% of participants in the 140-mg group and 62% in the 210-mg group. No participants in the placebo group demonstrated improvement of 75% or higher. The most common AEs were nasopharyngitis, upper respiratory tract infection, arthralgia, and injection-site erythema. Serious AEs reported during the study included renal colic (1 participant), ec-topic pregnancy (1 participant), and grade 3 asymptomatic neutropenia (2 participants). Both cases of neutropenia were noted at the first assessment after brodalumab initiation (week 2) and resolved when the study drug was withheld.17
Results for this new IL-17 blocker are encouraging, but phase 3 data of brodalumab will need to be awaited.
Tildrakizumab
Tildrakizumab is a humanized IgG1 monoclonal antibody that blocks the p19 subunit of IL-23. In a randomized, double-blind, phase 2b trial, 355 adults with moderate to severe psoriasis were randomized to receive subcutaneous injections of tildrakizumab (5 mg, 25 mg, 100 mg, or 200 mg) or placebo.18 In part 1 of the study, injections were administered at weeks 0 and 4. Part 2 of the study started at week 16. In part 2, responders with a 75% improvement in PASI score in the 5- and 25-mg groups continued their dose, while responders in the 100- or 200-mg groups were randomized again to continue the same dose or a reduced dose (100 mg to 25 mg; 200 mg to 100 mg) every 12 weeks from weeks 16 to 52. Those in the placebo group received tildrakizumab 25 mg every 12 weeks in part 2. The primary end point was the mean change in PASI score from baseline to week 16, which was significantly greater in all tildrakizumab groups than in the placebo group (P<.001 for all comparisons). Improvements of 75% in PASI score were achieved by 74% in the 200-mg group, 66% in the 100-mg group, 64% in the 25-mg group, and 33% in the 5-mg group. In contrast, 4.9% in the placebo group achieved an improvement of 75%. At week 52, no loss of efficacy was seen in those participants who had achieved 75% improvement in PASI score at week 16 and had continued their prior doses. The rates of AEs seen in the tildrakizumab groups were 60% to 71% compared to 69% in the placebo group. The most common AE was nasopharyngitis, occurring in 12% to 20% of participants in each group. Serious AEs were uncommon.18 Phase 3 studies are currently underway.19
Guselkumab
Guselkumab is a human IgG1 monoclonal antibody in clinical development that specifically blocks the p19 subunit of IL-23. In a double-blind, placebo-controlled, phase 1 study, 24 participants with moderate to severe plaque psoriasis were randomized to receive a single 10-mg (n=5), 30-mg (n=5), 100-mg (n=5), or 300-mg (n=5) dose of guselkumab or placebo (n=4).20 At week 12, 50% in the 10-mg group, 60% in both the 30- and 100-mg groups, and 100% in the 300-mg group showed 75% improvement in PASI score versus 0% in the placebo group. Improvements in PASI scores were generally maintained through week 24. The rates of AEs were 65% (13/20) in the combined guselkumab group and 50% (2/4) in the placebo group.20
Small Molecule Inhibitors
In contrast to biologics, which mainly target soluble cytokine or cellular receptors, small molecule inhibitors target enzymes within signaling pathways. Small molecule inhibitors have some advantages over biologics in that they are relatively inexpensive to produce and can be administered orally; thus, they may be preferred by some patients over injectable drugs. There are several agents that are undergoing clinical trials in psoriasis, including PDE4 inhibitors and Jak inhibitors.
Apremilast
Apremilast is an oral small molecule PDE4 inhibitor that was approved by the US Food and Drug Administration in March 2014 for the treatment of adult patients with active psoriatic arthritis; an indication for moderate to severe plaque psoriasis was approved in September 2014.21 Phosphodiesterase 4 is a cyclic adenosine monophosphate–specific phosphodiesterase inhibitor, which is dominant in inflammatory cells. Inhibition of PDE4 increases intracellular cyclic adenosine monophosphate levels, thus downregulating proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-12, and IL-23, and increasing the production of anti-inflammatory cytokines such as IL-10.22
Phase 2 and phase 3 studies have demonstrated the clinical efficacy of apremilast in the treatment of patients with moderate to severe plaque psoriasis. In a 16-week randomized, placebo-controlled, phase 3 trial (ESTEEM 2), 408 participants were randomized to receive oral apremilast 30 mg twice daily (n=275) or placebo (n=138).23 Improvement of 75% in PASI score was achieved by 29% of participants in the apremilast group at week 16. The most common AEs were diarrhea (16%) and nausea (18%), which were predominantly mild, occurring most commonly in the first week and resolving within 1 month. No cases of severe diarrhea or severe nausea were reported. Apremilast had no apparent effect on the results of hematological or serum chemistry tests.23 Although the US Food and Drug Administration warns of a possible link between apremilast and depression,24 data are mostly related to roflumilast, another PDE4 inhibitor. Studies in patients with chronic obstructive pulmonary disease have noted increased cases of depression (1.21% vs 0.82%) and suicidal ideation/attempt (0.03% vs 0.02%) in patients treated with roflumilast versus placebo.25
Jak Inhibitors
Janus kinases are a family of intracellular tyrosine kinases that connect several cytokine receptors to the signal transducer and activator of transcription pathways.26 There are 4 Jak family members: Jak1, Jak2, Jak3, and tyrosine kinase 2. Janus kinases 1 and 2 have roles in interferon signaling, while Jak3 transduces signals from IL-2, IL-7, IL-15, and IL-21, which are T-cell growth and survival factors.
Tofacitinib is a novel oral signal transduction molecule that blocks the Jak3 pathway. A phase 2b, 12-week, dose-ranging study was conducted to assess the efficacy and safety of 3 twice-daily regimens of tofacitinib versus placebo in patients with moderate to severe chronic plaque psoriasis.27 One hundred and ninety-seven participants were randomized to receive oral tofacitinib (2 mg, 5 mg, or 15 mg; n=49 each) or placebo (n=50) twice daily for 12 weeks with a 4-week follow-up period. The primary end point was the proportion of participants achieving at least a 75% reduction in PASI score at week 12 (25.0% with 2 mg, 40.8% with 5 mg, 66.7% with 15 mg, 2.0% with placebo). Similarly, a higher proportion of participants achieving 90% reduction in PASI score was seen at weeks 8 and 12 in all tofacitinib-treated participants versus placebo. The most common AEs were upper respiratory tract infection, sinusitis, nasopharyngitis, and headache. A number of changes in laboratory parameters occurred in the tofacitinib groups. Mild dose-related decreases in hemoglobin were noted at week 12 for all tofacitinib groups, and a small increase (mean, 0.04 mg/dL) in serum creatinine levels was observed in the 15-mg group. Decreases in neutrophil counts were observed with higher doses of tofacitinib, with the maximum mean decrease of 0.9×103/mm3 from baseline observed in the 15-mg group at week 4. After weeks 4 through 8, mean neutrophil counts began to return to baseline levels. Dose-related increases in total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were observed by week 2 and remained at this level through week 12; mean lipid levels decreased to baseline levels after cessation of active treatment. One participant in the 15-mg group developed an elevated alanine aminotransferase level that was more than 2.5 times the highest normal limit. Three participants experienced 5 serious AEs.27 These early results show that tofacitinib can be a safe and effective treatment in patients with psoriasis, but further data from phase 3 studies will need to be awaited.
Another Jak inhibitor under investigationfor the treatment of psoriasis is ruxolitinib, an inhibitor of Jak1 and Jak2, which has been primarily studied as a topical agent for milder cases of the disease.28
Conclusion
Many new drugs are currently on the horizon and will increase our armamentarium for treating psoriasis. Some of these agents promise greater levels of efficacy than currently used therapies. Although this review focuses on systemic agents, there also are a number of topical formulations in the pipeline. These new agents will certainly increase our options when choosing the most suitable treatment for a patient with psoriasis, but safety will remain a primary concern, and time and experience will tell whether efficacy outweighs any potential side effects.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
2. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 pt 1):401-407.
3. Shah R, Bewley A. Psoriasis: ‘the badge of shame.’ a case report of a psychological intervention to reduce and potentially clear chronic skin disease. Clin Exp Dermatol. 2014;39:600-603.
4. Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165-178.
5. Chi CC, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014;2014:862851.
6. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
7. Leonardi CL, Gordon KB. New and emerging therapies in psoriasis. Semin Cutan Med Surg. 2014;33(2, suppl 2):S37-S41.
8. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
9. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56.
10. Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461-467.
11. Adami S, Cavani A, Rossi F, et al. The role of interleukin-17A in psoriatic disease. BioDrugs. 2014;28:487-497.
12. Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72.
13. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
14. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
15. Krstic A, Mojsilovic S, Jovcic G, et al. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34-41.
16. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
17. Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
18. Langley RGB, Thaci D, Papp KA, et al. MK-3222, an anti–IL-23p19 humanized monoclonal antibody, provides significant improvement in psoriasis over 52 weeks of treatment that is maintained after discontinuation of dosing. Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8056.
19. Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:156-169.
20. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
21. Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016-2029.
22. van de Kerkhof PC. Apremilast: a step forward in the treatment of psoriasis? Lancet. 2012;380:708-709.
23. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2). Poster presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO. Poster 8412.
24. Otezla [product insert]. Summit, NJ: Celgene Corporation; 2014.
25. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
26. Palanivel JA, Macbeth AE, Chetty NC, et al. An insight into JAK-STAT signalling in dermatology. Clin Exp Dermatol. 2014;39:513-518.
27. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
28. Hsu L, Armstrong AW. JAK inhibitors: treatment efficacy and safety profile in patients with psoriasis. J Immunol Res. 2014;2014:283617.
Practice Points
- Secukinumab is an anti–IL-17 antibody approved for the treatment of psoriasis. It is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
- The new biological agents have shown promising results with some patients achieving psoriasis area and severity index scores of 90 and 100.
- A number of small molecule inhibitors also are in the pipeline, with apremilast the first one to have reached approval for psoriasis.
Manage Your Dermatology Practice: Selecting Cosmetic Procedures to Offer in Your Practice
Dermatologists with a strictly medical or surgical practice may consider offering cosmetic procedures to their patients. Dr. Gary Goldenberg provides tips on how to market your practice as cosmetic by obtaining patient input, estimating start-up costs, and determining which procedures may benefit patients with medical conditions such as acne and rosacea.
Dermatologists with a strictly medical or surgical practice may consider offering cosmetic procedures to their patients. Dr. Gary Goldenberg provides tips on how to market your practice as cosmetic by obtaining patient input, estimating start-up costs, and determining which procedures may benefit patients with medical conditions such as acne and rosacea.
Dermatologists with a strictly medical or surgical practice may consider offering cosmetic procedures to their patients. Dr. Gary Goldenberg provides tips on how to market your practice as cosmetic by obtaining patient input, estimating start-up costs, and determining which procedures may benefit patients with medical conditions such as acne and rosacea.
Manage Your Dermatology Practice: Managing Difficult Patient Encounters
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Manage Your Dermatology Practice: Attracting New Dermatology Patients
Attracting patients to your dermatology practice requires a multipronged approach. Dr. Gary Goldenberg discusses how technology and referrals will impact your patient base. Patient reviews also will affect your practice.
Attracting patients to your dermatology practice requires a multipronged approach. Dr. Gary Goldenberg discusses how technology and referrals will impact your patient base. Patient reviews also will affect your practice.
Attracting patients to your dermatology practice requires a multipronged approach. Dr. Gary Goldenberg discusses how technology and referrals will impact your patient base. Patient reviews also will affect your practice.
Manage Your Dermatology Practice: Mastering Communication With Patients About Their Expectations
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Communicating expectations to patients at their first visit and at follow-up is an important aspect of managing your dermatology practice. Dr. Gary Goldenberg discusses patient expectations for clinical conditions such as acne or psoriasis and for cosmetic treatments. He also advises what to do if you encounter a patient with unreasonable expectations.
Manage Your Dermatology Practice: Creating a Strong Online Presence
Managing a dermatology practice involves having a strong online presence that utilizes your practice's Web site and social media. Dr. Gary Goldenberg provides insight on creating content for your Web site, remaining HIPAA compliant, and keeping your social media posts relevant.
Managing a dermatology practice involves having a strong online presence that utilizes your practice's Web site and social media. Dr. Gary Goldenberg provides insight on creating content for your Web site, remaining HIPAA compliant, and keeping your social media posts relevant.
Managing a dermatology practice involves having a strong online presence that utilizes your practice's Web site and social media. Dr. Gary Goldenberg provides insight on creating content for your Web site, remaining HIPAA compliant, and keeping your social media posts relevant.
Filler Placement
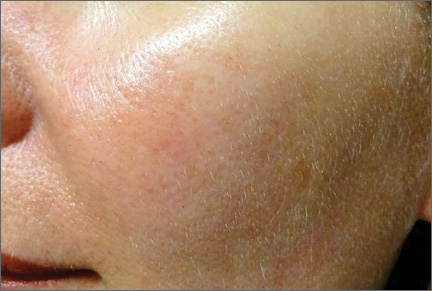
In a recently published article in the Journal of Cosmetic Dermatology (2014;13:91-98), Goodier et al compared low volume deep placement cheek injection and mid to deep dermal nasolabial fold injection for the correction of nasolabial folds with hyaluronic acid (HA) filler. In this split-face study, 3 injection techniques were utilized: (1) deep bolus injection into the mid to lateral cheek, (2) mid to deep dermal injection into the nasolabial fold, or (3) both techniques. Results were assessed in 4 to 6 weeks by a blinded investigator.
Globally, patients and investigators noted no statistical difference using the wrinkle severity score. All 3 techniques showed improvement. Patients preferred injection using both techniques, which was associated with the greatest amount of filler product injected. The authors concluded that injection of a dermal HA filler into either the nasolabial fold or mid to lateral cheek resulted in similar improvement for the correction of the nasolabial folds.
What’s the issue?
Although this study used a single HA agent, it showed that patients’ nasolabial folds improved using both techniques: deep depot placement in the cheeks and mid to deep dermal nasolabial fold injection. It may come as no surprise that patients in this study showed a slight preference for both techniques. Although the trend now is to add volume and not fill, a combination of these techniques may give the best outcomes. What do you use in your practice? A study comparing different HA fillers available or one comparing HA to non-HA products would be interesting. Which agents do you use in your practice for nasolabial fold correction?
In a recently published article in the Journal of Cosmetic Dermatology (2014;13:91-98), Goodier et al compared low volume deep placement cheek injection and mid to deep dermal nasolabial fold injection for the correction of nasolabial folds with hyaluronic acid (HA) filler. In this split-face study, 3 injection techniques were utilized: (1) deep bolus injection into the mid to lateral cheek, (2) mid to deep dermal injection into the nasolabial fold, or (3) both techniques. Results were assessed in 4 to 6 weeks by a blinded investigator.
Globally, patients and investigators noted no statistical difference using the wrinkle severity score. All 3 techniques showed improvement. Patients preferred injection using both techniques, which was associated with the greatest amount of filler product injected. The authors concluded that injection of a dermal HA filler into either the nasolabial fold or mid to lateral cheek resulted in similar improvement for the correction of the nasolabial folds.
What’s the issue?
Although this study used a single HA agent, it showed that patients’ nasolabial folds improved using both techniques: deep depot placement in the cheeks and mid to deep dermal nasolabial fold injection. It may come as no surprise that patients in this study showed a slight preference for both techniques. Although the trend now is to add volume and not fill, a combination of these techniques may give the best outcomes. What do you use in your practice? A study comparing different HA fillers available or one comparing HA to non-HA products would be interesting. Which agents do you use in your practice for nasolabial fold correction?
In a recently published article in the Journal of Cosmetic Dermatology (2014;13:91-98), Goodier et al compared low volume deep placement cheek injection and mid to deep dermal nasolabial fold injection for the correction of nasolabial folds with hyaluronic acid (HA) filler. In this split-face study, 3 injection techniques were utilized: (1) deep bolus injection into the mid to lateral cheek, (2) mid to deep dermal injection into the nasolabial fold, or (3) both techniques. Results were assessed in 4 to 6 weeks by a blinded investigator.
Globally, patients and investigators noted no statistical difference using the wrinkle severity score. All 3 techniques showed improvement. Patients preferred injection using both techniques, which was associated with the greatest amount of filler product injected. The authors concluded that injection of a dermal HA filler into either the nasolabial fold or mid to lateral cheek resulted in similar improvement for the correction of the nasolabial folds.
What’s the issue?
Although this study used a single HA agent, it showed that patients’ nasolabial folds improved using both techniques: deep depot placement in the cheeks and mid to deep dermal nasolabial fold injection. It may come as no surprise that patients in this study showed a slight preference for both techniques. Although the trend now is to add volume and not fill, a combination of these techniques may give the best outcomes. What do you use in your practice? A study comparing different HA fillers available or one comparing HA to non-HA products would be interesting. Which agents do you use in your practice for nasolabial fold correction?
Pros and Cons of Devices for Rosacea
For more information, access Dr. Goldenberg's article from the July 2014 issue, "Devices and Topical Agents for Rosacea Management."
For more information, access Dr. Goldenberg's article from the July 2014 issue, "Devices and Topical Agents for Rosacea Management."
For more information, access Dr. Goldenberg's article from the July 2014 issue, "Devices and Topical Agents for Rosacea Management."
Devices and Topical Agents for Rosacea Management
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.
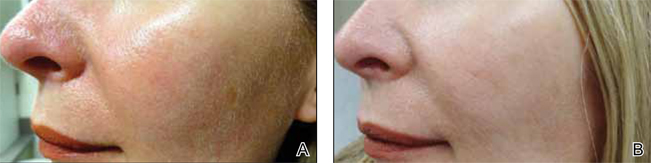
According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
Rosacea is a common chronic inflammatory disease that typically affects centrofacial skin, particularly the convexities of the forehead, nose, cheeks, and chin. Occasionally, involvement of the scalp, neck, or upper trunk can occur.1 Rosacea is more common in light-skinned individuals and has been called the “curse of the Celts,”2 but it also can affect Asian individuals and patients of African descent. Although rosacea affects women more frequently, men are more likely to develop severe disease with complications such as rhinophyma. Diagnosis is made on clinical grounds, and histologic confirmation rarely is necessary.
Despite its high incidence and recent advances, the pathogenesis of rosacea is still poorly understood. A combination of factors, such as aberrations in innate immunity,3 neurovascular dysregulation, dilated blood and lymphatic vessels, and a possible genetic predisposition seem to be involved.4 Presence of commensal Demodex folliculorum mites may be a contributing factor for papulopustular disease.
Patients can present with a range of clinical features, such as transient or persistent facial erythema, telangiectasia, papules, pustules, edema, thickening, plaque formation, and ocular manifestations. Associated burning and stinging also may occur. Rosacea-related erythema (eg, lesional and perilesional erythema) can be caused by inflammatory lesions or can present independent of lesions in the case of diffuse facial erythema. Due to the diversity of clinical signs and limited knowledge regarding its etiology, rosacea is best regarded as a syndrome and has been classified into 4 subtypes—erythematotelangiectatic, papulopustular, phymatous, and ocular—and 1 variant (granulomatous rosacea).5 The most common phymatous changes affect the nose, with hypertrophy and lymphedema of subcutaneous tissues. Other sites that can be affected are the ears, forehead, and chin. Ocular manifestations affect approximately 50% of rosacea patients,6 ranging from conjunctivitis and blepharitis to keratitis and corneal ulceration, thereby requiring ophthalmologic assessment.
Because rosacea affects facial appearance, it can have a devastating impact on the patient’s quality of life, leading to social isolation. Although there is no cure available for rosacea, lifestyle modification and treatment can reduce or control its features, which tend to exacerbate and remit. There are a number of possible triggers for rosacea that ideally should be avoided such as sun exposure, hot or cold weather, heavy exercise, emotional stress, and consumption of alcohol and spicy foods. It is essential to consider disease subtype as well as the signs and symptoms presenting in each individual patient when approaching therapy selection. Most well-established US Food and Drug Administration (FDA)–approved treatments of rosacea target the papulopustular aspect of disease, including the erythema associated with the lesions. These treatments include topical and systemic antibiotics and azelaic acid. Non–FDA-approved agents such as topical and systemic retinoids, topical calcineurin inhibitors, and topical benzoyl peroxide also are used, though there is limited evidence of their efficacy.7
Management options for diffuse facial erythema and telangiectasia, however, are limited. Standard rosacea treatments often are not efficacious in treating these aspects of the disease, thereby requiring an alternative approach. This article reviews devices and topical agents currently available for the management of rosacea.
Skin Care
The skin of rosacea patients often is sensitive and prone to irritation; therefore, a good skin care regimen is an integral part of disease management and should include a gentle cleanser, moisturizer, and sunscreen.8 Lipid-free liquid cleansers or synthetic detergent (syndet) cleansers with a neutral to slightly acidic pH (ie, similar to the pH of normal skin) are ideal.9 Following cleansing, the skin should be gently dried. It may be beneficial to wait up to 30 minutes before application of a moisturizer to avoid irritation. Hydrating moisturizers should be free of irritants or abrasives, allowing maintenance of stratum corneum pH in an acid range of 4 to 6. Green-tinted makeup can be a useful tool in covering areas of erythema.
Devices
A variety of devices targeting hemoglobin are reported to be effective for the management of erythema and telangiectasia in rosacea patients, including the 595-nm pulsed dye laser (PDL), the potassium titanyl phosphate (KTP) laser, the 1064-nm Nd:YAG laser, and noncoherent intense pulsed light (IPL) sources.
The major chromophore in blood vessels is oxyhemoglobin, with 2 major absorption bands in the visible light spectrum at 542 and 577 nm. There also is notable albeit lesser absorption in the near-infrared range from 700 to 1100 nm.10 Following absorption by oxyhemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel causing photocoagulation, mechanical injury, and finally thrombosis.
Pulsed Dye Laser (585–595 nm)
Among the vascular lasers, the PDL has a long safety record. It was the first laser that used the concept of selective photothermolysis for treatment of vascular lesions.11,12 The first PDLs had a wavelength of 577 nm, while current PDLs have wavelengths of 585 or 595 nm with longer pulse durations and circular or oval spot sizes that are ideal for treatment of dermal vessels. The main disadvantage of PDLs is the development of posttreatment purpura. The longer pulse durations of KTP lasers avoid damage to cutaneous vasculature and eliminate the risk for bruising. Nonetheless, the wavelength of the PDL provides a greater depth of penetration due to its substantial absorption by cutaneous vasculature compared to the shorter wavelength of the KTP laser.
Although newer-generation PDLs still have the potential to cause purpura, various attempts have been made to minimize this risk, such as the use of longer pulse durations, multiple minipulses or “pulselets,”13 and multiple passes. Separate parameters may need to be used when treating linear vessels and diffuse erythema, with longer pulse durations required for larger vessels. The Figure shows a rosacea patient with facial telangiectasia before and after 1 treatment with a PDL.

According to Alam et al,14 purpuric settings were more efficacious in a comparison of variable-pulsed PDLs for facial telangiectasia. In 82% (9/11) of cases, greater reduction in telangiectasia density was noted on the side of the face that had been treated with purpuric settings versus the other side of the face.14 Purpuric settings are particularly effective in treating larger vessels, while finer telangiectatic vessels may respond to purpura-free settings.
In a study of 12 participants treated with a 595-nm PDL at a pulse duration of 6 ms and fluences from 7 to 9 J/cm2, no lasting purpura was seen; however, while 9 participants achieved more than 25% improvement after a single treatment, only 2 participants achieved more than 75% improvement.15 Nonetheless, some patients may prefer this potentially less effective treatment method to avoid the socially embarrassing side effect of purpura.
In a study of 12 rosacea patients, a 75% reduction in telangiectasia scores was noted after a mean of 3 treatments with the 585-nm PDL using 450-ms pulse durations. Purpura occurred in all patients.16 In another study by Madan and Ferguson,17 18 participants with nasal telangiectasia that had been resistant to the traditional round spot, 595-nm PDL and/or 532-nm KTP laser were treated with a 3x10-mm elliptical spot, ultra-long pulse, 595-nm PDL with a 40-ms pulse duration and double passes. Complete clearance was seen in 10 (55.6%) participants and 8 (44.4%) showed more than 80% improvement. No purpura was associated with the treatment.17
Further studies comparing the efficacy of nonpurpuric and purpuric settings in the same patient would allow us to determine the most effective option for future treatment.
KTP Laser (532 nm)
Potassium titanyl phosphate lasers have the disadvantage of higher melanin absorption, which can lead to epidermal damage with postinflammatory hyperpigmentation. Their use is limited to lighter skin types. Because of its shorter wavelength, the KTP laser is best used to treat superficial telangiectasia. The absence of posttreatment purpura can make KTP lasers a popular alternative to PDLs.17 Uebelhoer et al18 performed a split-face study in 15 participants to compare the 595-nm PDL and 532-nm KTP laser. Although both treatments were effective, the KTP laser achieved 62% clearance after the first treatment and 85% clearance 3 weeks after the third treatment compared to 49% and 75%, respectively, for the PDL. Interestingly, the degree of swelling and erythema posttreatment were greater on the KTP laser–treated side.18
Nd:YAG (1064 nm)
The wavelength of the Nd:YAG laser targets the lower absorption peak of oxyhemoglobin. In a study of 15 participants with facial telangiectasia who were treated with a 1064-nm Nd:YAG laser at day 0 and day 30 using a 3-mm spot size, a fluence of 120 to 170 J/cm,2 and 5- to 40-ms pulse durations, 73% (11/15) showed moderate to significant improvement at day 0 and day 30 and 80% improvement at 3 months’ follow-up.19 In a split-face study of 14 patients, treatment with the 595-nm PDL with a fluence of 7.5 J/cm2, pulse duration of 6 ms, and spot size of 10 mm was compared with the 1064-nm Nd:YAG laser with a fluence of 6 J/cm2, pulse duration of 0.3 ms, and spot size of 8 mm.20 Erythema improved by 6.4% from baseline on the side treated with the PDL. Although participants rated the Nd:YAG laser treatment as less painful, they were more satisfied with the results of the PDL treatment.20 In another split-face study comparing the 595-nm PDL and 1064-nm Nd:YAG laser, greater improvement was reported with the Nd:YAG laser, though the results were not statistically significant.21
Intense Pulsed Light
While lasers use selective photothermolysis, IPL devices emit noncoherent light at a wavelength of 500 to 1200 nm. Cutoff filters allow for selective tissue damage depending on the absorption spectra of the tissue. Longer wavelengths are effective for the treatment of deeper vessels, while shorter wavelengths target more superficial vessels; however, the shorter wavelengths can interact with melanin and should be avoided in darker skin types. In a phase 3 open trial, 34 participants were treated with IPL with a 560-nm cutoff filter and fluences of 24 to 32 J/cm2. The mean reduction of erythema following 4 treatments was 39% on the cheeks and 22% on the chin; side effects were minimal.22
Photodynamic Therapy
Photodynamic therapy is an effective and widely used treatment method for a number of skin conditions. Following its success in the treatment of acne, it also has been used in the management of rosacea, though the exact mechanism of action remains unclear.
Photodynamic therapy involves topical application of a photosensitizing agent (eg, 5-aminolevulinic acid, methyl aminolevulinate [MAL]) followed by exposure to red or blue light. The photosensitizing agent accumulates semiselectively in abnormal skin tissue and is converted to protoporphyrin IX, which induces a toxic skin reaction through reactive oxygen radicals in the presence of visible light.23 Photodynamic therapy generally is well tolerated. The primary side effects are pain, burning, and stinging.
In 3 of 4 (75%) patients treated with MAL and red light, rosacea clearance was noted after 2 to 3 sessions. Remission lasted for 3 months in 2 (66.7%) participants and for 9 months in 1 (33.3%) participant.24 In another study, 17 patients were treated with MAL and red light. Results were good in 10 participants (58.8%), fair in 4 (23.5%), and poor in 3 (17.6%).23
ALPHA-Adrenergic Receptor Agonists
Recently, the α-adrenergic receptor agonists brimonidine tartrate and oxymetazoline have been found to be effective in controlling diffuse facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature. Brimonidine tartrate is a potent α2-agonist that is mainly used for treatment of open-angle glaucoma. In 2 phase 3 controlled studies, once-daily application of brimonidine tartrate gel 0.5% was found to be effective and safe in reducing the erythema of rosacea.25 Brimonidine tartrate gel is the first FDA-approved treatment of facial erythema associated with rosacea. Possible side effects are erythema worse than baseline (4%), flushing (3%), and burning (2%).26 Oxymetazoline is a potent α1- and partial α2-agonist that is available as a nasal decongestant. Oxymetazoline solution 0.05% used once daily has been shown in case reports to reduce rosacea-associated erythema for several hours.27
Nicotinamide
Nicotinamide is the amide form of niacin, which has both anti-inflammatory properties and a stabilizing effect on epidermal barrier function.28 Although topical application of nicotinamide has been used in the treatment of inflammatory dermatoses such as rosacea,28,29 niacin can lead to cutaneous vasodilation and thus flushing. It has been hypothesized to potentially enhance the effect of PDL if used as pretreatment for rosacea-associated erythema.30
Conclusion
Rosacea can have a substantial impact on patient quality of life. Recent advances in treatment options and rapidly advancing knowledge of laser therapy are providing dermatologists with powerful tools for rosacea clearance. Lasers and IPL are effective treatments of the erythematotelangiectatic aspect of the disease, and careful selection of devices and treatment parameters can reduce unwanted side effects.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
- Ayres S Jr. Extrafacial rosacea is rare but does exist. J Am Acad Dermatol. 1987;16:391-392.
- Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med. 1997;90:144-150.
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12-15.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6, suppl 1):S15-S26.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6, suppl 1):S42-S43.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Draelos ZD. The effect of Cetaphil gentle skin cleanser on the skin barrier of patients with rosacea. Cutis. 2006;77:27-33.
- Hare McCoppin HH, Goldberg DJ. Laser treatment of facial telangiectases: an update. Dermatol Surg. 2010;36:1221-1230.
- Garden JM, Polla LL, Tan OT. The treatment of port-wine stains by the pulsed dye laser. analysis of pulse duration and long-term therapy. Arch Dermatol. 1988;124:889-896.
- Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263-276.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40:233-239.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-684.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med Sci. 2002;17:26-33.
- Madan V, Ferguson J. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers Med Sci. 2010;25:151-154.
- Uebelhoer NS, Bogle MA, Stewart B, et al. A split-face comparison study of pulsed 532-nm KTP laser and 595-nm pulsed dye laser in the treatment of facial telangiectases and diffuse telangiectatic facial erythema. Dermatol Surg. 2007;33:441-448.
- Sarradet DM, Hussain M, Goldberg DJ. Millisecond 1064-nm neodymium:YAG laser treatment of facial telangiectases. Dermatol Surg. 2003;29:56-58.
- Alam M, Voravutinon N, Warycha M, et al. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: a double-blind randomized controlled trial. J Am Acad Dermatol. 2013;69:438-443.
- Salem SA, Abdel Fattah NS, Tantawy SM, et al. Neodymium-yttrium aluminum garnet laser versus pulsed dye laser in erythemato-telangiectatic rosacea:comparison of clinical efficacy and effect on cutaneoussubstance (P) expression. J Cosmet Dermatol. 2013;12:187-194.
- Papageorgiou P, Clayton W, Norwood S, et al. Treatment of rosacea with intense pulsed light: significant improvement and long-lasting results. Br J Dermatol. 2008;159:628-632.
- Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199-1202.
- Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211:135-138.
- Fowler J, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- Routt ET, Levitt JO. Rebound erythema and burning sensation from a new topical brimonidine tartrate gel 0.33%. J Am Acad Dermatol. 2014;70:E37-E38.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Draelos ZD, Ertel K, Berge C. Niacinamide-containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135-141.
- Draelos ZD, Ertel KD, Berge CA. Facilitating facial retinization through barrier improvement. Cutis. 2006;78:275-281.
- Kim TG, Roh HJ, Cho SB, et al. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea-associated erythema by 585-nm pulsed dye laser in Koreans: a randomized, prospective, split-face trial. Br J Dermatol. 2011;164:573-579.
Practice Points
- Rosacea patients should be advised on appropriate skin care.
- Purpuric settings of the pulsed dye laser may be more effective in treating rosacea-associated erythema.
- Topical brimodine tartrate can control facial erythema, but patients should be warned of the potential risk for rebound erythema.