User login
Use of ECMO in the management of influenza-associated ARDS
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
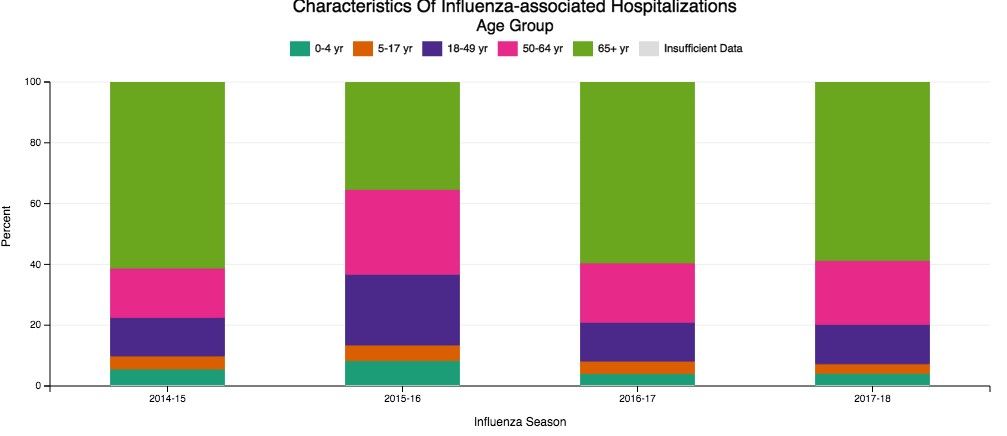
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.
Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.

Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.

Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.

