User login
A paraneoplastic potassium and acid-base disturbance
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
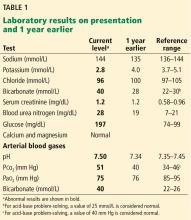
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
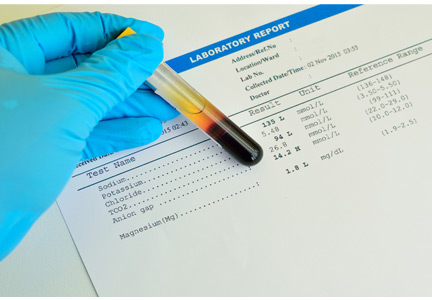
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
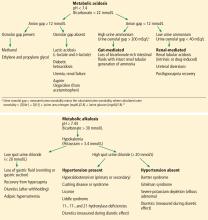
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
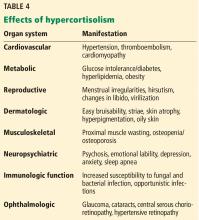
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
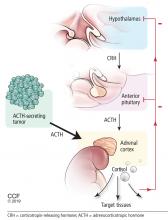
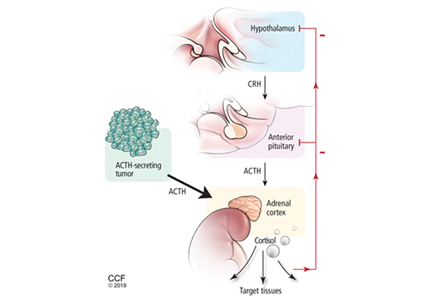
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
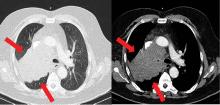
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
NOTE: The scenario presented here is partly based on cases reported elsewhere by Martínez-Valles et al1 and Fernández-Rodríguez et al.2
A 55-year-old man is admitted to the hospital with generalized malaise, paresthesias, and severe hypertension. He says he had experienced agitation along with weakness on exertion 24 hours before presentation to the emergency department, with subsequent onset of paresthesias in his lower extremities and perioral area.
He is already known to have mild chronic obstructive pulmonary disease, with a ratio of forced expiratory volume in 1 second (FEV1)to forced vital capacity (FVC) of less than 70% and an FEV1 85% of predicted. In addition, he was recently diagnosed with diabetes, resistant hypertension requiring maximum doses of 3 agents (a calcium channel blocker, an angiotensin-converting enzyme inhibitor, and a loop diuretic), and hyperlipidemia.
He is a current smoker with a 30-pack-year smoking history. He does not use alcohol. His family history is noncontributory.
ASSESSING ACID-BASE DISORDERS
1. What type of acid-base disorder does this patient have?
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
The patient has metabolic alkalosis.
A 5-step approach
1. Acidosis or alkalosis? The patient’s arterial pH is 7.5, which is alkalemic because it is higher than 7.44.
2. Metabolic or respiratory? The primary process in our patient is overwhelmingly metabolic, as his partial pressure of carbon dioxide (Pco2) is slightly elevated, a direction that would cause acidosis, not alkalosis.
3. The anion gap (the serum sodium concentration minus the sum of the chloride and bicarbonate concentrations) is normal at 8 mmol/L (DRG:HYBRiD-XL Immunoassay and Clinical Chemistry Analyzer, reference range 8–16).
4. Is the disturbance compensated? We have determined that this patient has a metabolic alkalemia; the question now is whether there is any compensation for the primary disturbance.
In metabolic alkalosis, the Pco2 may increase by approximately 0.6 mm Hg (range 0.5–0.8) above the nominal normal level of 40 mm Hg for each 1-mmol/L increase in bicarbonate above the nominal normal level of 25 mmol/L.4 If the patient requires oxygen, the calculation may be unreliable, however, as hypoxemia may have an overriding influence on respiratory drive.
Patients with chronically high Pco2 levels such as those with chronic obstructive pulmonary disease can become accustomed to high carbon dioxide levels and lose their hyper-
capnic respiratory drive. Giving oxygen supplementation is thought to decrease respiratory drive in these patients, so that they will breathe slower and retain more carbon dioxide. There is some degree of respiratory compensation for metabolic alkalosis that occurs by breathing less, though it is limited overall—even in very alkalotic patients, breathing less results in CO2 retention, which, by displacing O2 molecules in the alveoli, will in turn result in hypoxia. The brain then senses the hypoxia and makes one breathe faster, thereby limiting this compensation.
This patient’s serum bicarbonate level is 40 mmol/L, or 15 mmol/L higher than the nominal normal level. If he is compensating, his Pco2 should be 40 + (15 × 0.6) = 49 mm Hg, and in fact it is 51 mm Hg, which is within the normal range of expected compensation (47.5–52 mm Hg). Therefore, yes, he is compensating for the primary disturbance.
5. In metabolic acidosis, is there a delta gap? As our patient has metabolic alkalosis, not acidosis, this question does not apply in this case.
WHICH TEST TO FIND THE CAUSE?
2. Which is the best test to order next to determine the cause of this patient’s hypokalemic metabolic alkalosis?
- Serum magnesium level
- Spot urine chloride
- Renal ultrasonography
- 24-hour urine collection for sodium, potassium, and chloride
The patient’s loop diuretic is withheld for 12 hours and a spot urine chloride is obtained, which is reported as 44 mmol/L. This high value suggests that a volume-independent hypokalemic metabolic alkalosis is present with potassium depletion.
As for the other answer choices:
Serum magnesium. Though hypomagnesemia can cause hypokalemia due to lack of inhibition of renal outer medullary potassium channels and subsequent increased excretion of potassium in the apical tubular membrane, it is not independently associated with acid-base disturbances.5
Renal ultrasonography gives information about structural kidney disease but is of limited utility in identifying the cause of hypokalemic metabolic alkalosis.
A 24-hour urine collection is unnecessary in this setting and would ultimately result in delay in diagnosis, as spot urine chloride is a more efficient means of rapidly distinguishing volume-responsive vs volume-independent causes of hypokalemic metabolic alkalosis.6
IS HIS HYPERTENSION SECONDARY? IF SO, WHAT IS THE CAUSE?
Several features of this case suggest that the patient’s hypertension is secondary rather than primary. It is of recent onset. The patient’s family history is noncontributory, and his hypertension is resistant to the use of maximum doses of 3 antihypertensive agents.
3. Which of the following causes of secondary hypertension is not commonly associated with hypokalemia and metabolic alkalosis?
- Hyperaldosteronism
- Liddle syndrome
- Cushing syndrome
- Renal parenchymal disease
- Chronic licorice ingestion
Renal parenchymal disease is a cause of resistant hypertension, but it is not characterized by metabolic alkalosis, hypokalemia, and elevated urine chloride,7 while the others listed here—hyperaldosteronism, Liddle syndrome, Cushing syndrome, and chronic licorice ingestion—are. Other common causes of resistant hypertension without these metabolic abnormalities include obstructive sleep apnea, alcohol abuse, and nonadherence to treatment.
While treatment of hypertension with loop diuretics can result in hypokalemia and metabolic alkalosis due to the effect of these drugs on potassium reabsorption in the loop of Henle, the patient’s hypokalemia persisted after this agent was withdrawn.8
Causes of hypokalemic metabolic alkalosis with and without hypertension are further delineated in Figure 1.
Additional diagnostic testing: Plasma renin and plasma aldosterone
At this juncture, the differential diagnosis for this patient’s potassium depletion, metabolic alkalosis, high urine chloride, and hypertension has been narrowed to primary or secondary hyperaldosteronism, surreptitious mineralocorticoid ingestion, Cushing syndrome, licorice ingestion, Liddle syndrome, or one of the 3 hydroxylase deficiencies (11-, 17-, and 21-) (Figure 1).
Although clues in the history, physical examination, and imaging may suggest a specific cause of his abnormal laboratory values, the next step in the diagnostic workup is to measure the plasma renin and aldosterone levels (Table 3).
HYPERALDOSTERONISM
4. Hyperaldosteronism is associated with which of the following patterns of renin and aldosterone values?
- High renin, high aldosterone, normal ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA)
- Low renin, low aldosterone, normal PAC–PRA ratio
- Low renin, high aldosterone, high PAC–PRA ratio
- High renin, low aldosterone, low PAC–PRA ratio
The pattern of low renin, high aldosterone, and high PAC–PRA ratio is associated with hyperaldosteronism.
Primary hyperaldosteronism
Primary hyperaldosteronism is one of the most common causes of resistant hypertension and is underappreciated, being diagnosed in up to 20% of patients referred to hypertension specialty clinics.7 Potassium levels may be normal, likely contributing to its lack of recognition in this target population.
Primary hyperaldosteronism should be suspected in patients who have a plasma aldosterone PAC–PRA ratio greater than 20 with elevated plasma aldosterone concentrations
(> 15 ng/dL).
Persistently elevated aldosterone levels in the setting of elevated plasma volume is proof that aldosterone secretion is independent of the renin-angiotensin-aldosterone axis, and therefore is autonomous (secondary to adrenal tumor or hyperplasia). Further testing in the form of oral salt loading, saline infusion, or fludrocortisone (a sodium-retaining steroid) administration is thus required to confirm inappropriate, autonomous aldosterone secretion.9
After establishing the diagnosis of primary hyperaldosteronism, one should determine the subtype (ie, due to an adrenal carcinoma, unilateral hypersecreting adenoma, or unilateral or bilateral hyperplasia). Further testing includes adrenal computed tomography (CT) to rule out adrenal carcinomas, which are suspected with adenomas larger than 4 cm. Though part of the diagnostic workup, CT as a means of confirmational testing alone does not preclude the possibility of bilateral adrenal hyperplasia in some patients, even in the presence of an adrenal adenoma. For this reason, adrenal venous sampling is required to definitively determine whether the condition is due to a hypersecreting adrenal adenoma or unilateral or bilateral hyperplasia.9,10
Treatment of primary hyperaldosteronism depends on the subtype of the disease and involves salt restriction in addition to an aldosterone antagonist (spironolactone or eplerenone in the case of bilateral disease) or surgery (unilateral disease).9,11,12
Secondary hyperaldosteronism
Secondary hyperaldosteronism should be suspected when plasma renin and aldosterone levels are both elevated with a PAC–PRA ratio less than 10.
This pattern is most commonly seen with diuretic use but can also be a consequence of renal artery stenosis or, rarely, a renin-secreting tumor.13 Renal artery stenosis is a common finding in patients with hypertension undergoing cardiac catheterization, which is not surprising as more than 90% of such stenoses are atherosclerotic.7 Renin-secreting tumors are exceedingly rare, with fewer than 100 cases reported in the literature, and are more common in younger individuals.13
Our patient has low-normal aldosterone and plasma renin
On further testing, this patient’s plasma aldosterone level is 2.55 ng/dL (normal < 15 ng/dL), his plasma renin activity is 0.53 ng/mL/hour (normal 0.2–2.8 ng/mL/hour), and his PAC–PRA ratio is therefore 4.81.
The categories discussed thus far have included primary and secondary hyperaldosteronism, which typically do not present with low to normal levels of both renin and aldosterone. Surreptitious mineralocorticoid use could present in this manner, but is unlikely in this patient, whose medications do not include fludrocortisone.
The low-normal values thus lead to consideration of a third category: apparent mineralocorticoid excess. Diseases in this category such as Cushing disease or adrenocorticotropic hormone (ACTH) excess are characterized by increases in corticosteroids so that the potassium depletion, metabolic alkalosis, and hypertension are not a consequence of renin and aldosterone but rather the excess corticosteroids.14
Causes of apparent mineralocorticoid excess
There are several possible causes of mineralocorticoid excess associated with hypertension and hypokalemic metabolic alkalosis not due to renin and aldosterone.
Chronic licorice ingestion in high volumes is one such cause and is thought to result in inhibition of 11B-hydroxysteroid dehydrogenase or possibly cortisol oxidase by licorice’s active component, glycyrrhetinic acid. This inhibition results in an inability to convert cortisol to cortisone. The cortisol excess binds to mineralocorticoid receptors, and acting like aldosterone, results in hypertension and hypokalemic metabolic alkalosis as well as feedback inhibition of renin and aldosterone levels.15
Partial hydroxylase deficiencies, though rare, should also be considered as a cause of hypokalemic metabolic alkalosis, hypertension, and, potentially, hirsutism and clitoromegaly in women. They can be diagnosed with elevated levels of 17-ketosteroids and dehydroepiandrosterone sulfate, both of which, in excess, may act on aldosterone receptors in a manner similar to cortisol.16
Liddle syndrome, a rare autosomal dominant condition, may also present with suppressed levels of both renin and aldosterone. In contrast to the disorders of nonaldosterone mineralocorticoid excess, however, the sodium channel defect in Liddle syndrome is characterized by a primary increase in sodium reabsorption in the collecting tubule and potassium wasting. The resultant volume expansion leads to suppressed renin and aldosterone levels and hypertension with low potassium and elevated bicarbonate concentrations.17
Liddle syndrome is commonly diagnosed in childhood but may go unrecognized due to occasional absence of hypokalemia at presentation. Potassium-sparing diuretics such as amiloride or triamterene are the mainstays of treatment.18
Rates of cardiovascular and all-cause mortality are increased in patients with long-term hypercortisolism, even after plasma concentrations of cortisol are normalized.21
Figure 2 shows the cascade of the hypothalamic-pituitary-adrenal axis.
TESTING FOR HYPERCORTISOLISM IN OUR PATIENT
Given the patient’s clinical presentation and laboratory and imaging findings with normal plasma renin and aldosterone levels, a workup for suspected hypercortisolism is initiated.
Initial diagnostic testing for hypercortisolism depends on the degree of clinical suspicion. In those with low probability of the disease, testing should consist of 1 of the following, as a single negative test may be sufficient to rule out the disease:
- 24-hour urinary cortisol levels
- Overnight dexamethasone suppression testing
- Late-night salivary cortisol measurements.
In those with a high index of suspicion, 2 of the aforementioned tests should be performed, as 1 normal result may not be sufficient to exclude the diagnosis.22,23
A 24-hour urinary cortisol collection and overnight dexamethasone suppression test are obtained. His 24-hour urinary free cortisol level is elevated at 6,600 µg (normal 4–100), and suppression testing with 8 mg of dexamethasone (a form of “high-dose” testing)demonstrates only an 8% decline in serum cortisol levels. Cortisol should generally drop more than 90%.
Morning serum cortisol concentration is less than 5 µg/dL (140 nmol/L) in most patients with Cushing disease (ie, a pituitary tumor), and is usually undetectable in normal subjects. Only about 50% of neuroendocrine ACTH-secreting tumors will suppress with this test.
The patient’s clinical presentation, in conjunction with his diagnostic testing, are thus consistent with Cushing syndrome.
CUSHING SYNDROME
Cushing syndrome is most often exogenous or iatrogenic, ie, a result of supraphysiologic doses of glucocorticoids used to treat a variety of inflammatory, autoimmune, and neoplastic conditions.
Endogenous Cushing syndrome, on the other hand, is rare, with an estimated prevalence of 0.7 to 2.4 cases per million per year. ACTH-dependent causes account for 80% of endogenous Cushing syndrome cases, with ACTH-secreting pituitary adenomas (Cushing disease) accounting for 75% to 80% and ectopic ACTH secretion accounting for 15% to 20%. Less than 1% of cases are due to tumors that produce corticotropin-releasing hormone (CRH).
ACTH-independent Cushing syndrome is diagnosed in 20% of endogenous cases and is most commonly caused by a unilateral adrenal tumor. Rare causes of ACTH-independent disease include adrenal carcinoma, McCune-Albright syndrome, and adrenal hyperplasia.24
The patient’s ACTH is high
To determine whether this is an ACTH-dependent or independent process, the next step is to order an ACTH level. His ACTH level is high at 107 pg/mL (normal < 46 pg/mL), confirming the diagnosis of ACTH-dependent Cushing syndrome.
To find out if this ACTH-dependent process is due to a pituitary adenoma, magnetic resonance imaging (MRI) of the pituitary is obtained but is normal.
Large masses (> 6 mm) strongly suggest Cushing disease, but these tumors are often small and may be missed even with more advanced imaging techniques. Corticotropin-secreting adenomas arising from normal cells in the pituitary retain some sensitivity to glucocorticoid negative feedback and CRH stimulation, and thus high-dose dexamethasone suppression testing in conjunction with CRH stimulation testing can be used to differentiate Cushing disease from ectopic ACTH secretion.24,25 Both of these tests have poor diagnostic accuracy, however, and thus inferior petrosal sampling remains the gold standard for the diagnosis of Cushing disease.
ACTH-SECRETING TUMORS
5. Cushing syndrome due to ectopic ACTH secretion is most commonly attributed to which of the following tumors?
- Small-cell lung carcinoma
- Pancreatic carcinoma
- Medullary thyroid carcinoma
- Gastrinoma
Severe cases of Cushing syndrome are often attributable to ectopic ACTH secretion due to an underlying malignancy, most commonly small-cell lung carcinoma or neuroendocrine tumors of pulmonary origin. Other causes include pancreatic and thymic neuroendocrine tumors, gastrinomas, and medullary thyroid carcinoma.25,26
Because most ACTH-producing tumors are intrathoracic, initial imaging in cases of suspected ectopic ACTH secretion should focus on the chest, with CT the usual first choice. Octreotide scintigraphy can also be useful in localizing disease, as many neuroendocrine tumors express somatostatin receptors. Specialized positron-emission tomography scans may also be helpful in tumor identification.24
TREATMENT OF CUSHING SYNDROME DUE TO ECTOPIC ACTH SECRETION
6. Which of the following is most appropriate medical therapy for suppression of cortisol secretion in Cushing syndrome due to ectopic ACTH secretion?
- Spironolactone
- Dexamethasone
- Somatostatin
- Estrogen
- Ketoconazole
Hyperglycemia, hypokalemia, hypertension, psychiatric disturbances, venous thromboembolism, and systemic infections appear to be common in ectopic ACTH syndrome and often correlate with the degree of hypercortisolemia. Severe Cushing syndrome due to ectopic ACTH secretion is an emergency requiring prompt control of cortisol secretion.
First-line treatments include steroidogenesis inhibitors (ketoconazole, metyrapone, etomidate, mitotane) and glucocorticoid receptor antagonists (mifepristone). High-dose spironolactone and eplerenone can also be used to treat the hypertension and hypokalemia associated with mineralocorticoid receptor stimulation. Definitive treatment involves surgical resection, chemotherapy, or radiotherapy when applicable.24,25
After confirmation of the diagnosis, the patient is prescribed ketoconazole and spironolactone, with substantial improvement. He subsequently is started on combination chemotherapy and radiation therapy for his small-cell lung carcinoma.
DISCUSSION
The differential diagnosis for hypokalemia is broad and relies on information obtained during the history and physical examination, followed by interpretation of selected laboratory results. Myriad pathologies in diverse organ systems, eg, diarrhea, renal tubular acidosis, and adrenal disease, may be responsible for a low serum potassium. Further categorizing potassium depletion on the basis of an associated acid-base disturbance, such as metabolic alkalosis, allows one to use an algorithmic approach that can identify specific etiologies responsible for both the potassium and the acid-base disturbances.
Using the spot urine chloride in the setting of hypokalemic metabolic alkalosis with or without hypertension can narrow the differential diagnosis and allow additional clinical findings to guide clinical problem-solving and decision-making, even for conditions not commonly encountered in routine medical practice.
Obtaining renin and aldosterone measurements in patients with potassium depletion, metabolic alkalosis, high urine chloride excretion, and hypertension permits further categorization into 3 clinical groups: elevated aldosterone and renin (secondary hyperaldosteronism), elevated aldosterone and low renin (primary hyperaldosteronism), or apparent mineralocorticoid excess wherein neither renin nor aldosterone are responsible for the syndrome.
The patient in our case had apparent mineralocorticoid excess as a consequence of an ACTH-producing small-cell carcinoma.
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
- Martínez-Valles MA, Palafox-Cazarez A, Paredes-Avina JA. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J 2009; 2:6174. doi:10.4076/1757-1626-2-6174
- Fernández-Rodríguez E, Villar-Taibo R, Pinal-Osorio I, et al. Severe hypertension and hypokalemia as first clinical manifestations in ectopic Cushing’s syndrome. Arq Bras Endocrinol Metabol 2008; 52(6):1066–1070. pmid:18820819
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84(1):27–34. doi:10.3949/ccjm.84a.16042
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21(6):920–923. doi:10.1681/ASN.2009121211
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18(10):2649–2652. doi:10.1681/ASN.2007070792
- Rose BD. Metabolic alkalosis. In: Clinical Physiology of Acid-Base and Electrolyte Disorders. 4th ed. New York, NY: McGraw-Hill, Health Professions Division; 1994:515.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117(25):e510–e526. doi:10.1161/CIRCULATIONAHA.108.189141
- Koeppen BM, Stanton BA. Physiology of diuretic action. In: Renal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc; 2013:167–178.
- Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med 1994; 121(11):877–885. pmid:7978702
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151(5):329–337. pmid:19721021
- Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008; 9(4):509–515. doi:10.1517/14656566.9.4.509
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135(4):258–261. pmid:11511140
- Haab F, Duclos JM, Guyenne T, Plouin PF, Corvol P. Renin secreting tumors: diagnosis, conservative surgical approach and long-term results. J Urol 1995; 153(6):1781–1784. pmid:7752315
- Sabbadin C, Armanini D. Syndromes that mimic an excess of mineralocorticoids. High Blood Press Cardiovasc Prev 2016; 23(3):231–235. doi:10.1007/s40292-016-0160-5
- Apostolakos JM, Caines LC. Apparent mineralocorticoid excess syndrome: a case of resistant hypertension from licorice tea consumption. J Clin Hypertens (Greenwich) 2016; 18(10):991–993. doi:10.1111/jch.12841
- Glatt K, Garzon DL, Popovic J. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Spec Pediatr Nurs 2005; 10(3):104–114. doi:10.1111/j.1744-6155.2005.00022.x
- Findling JW, Raff H, Hansson JH, Lifton RP. Liddle’s syndrome: prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997; 82(4):1071–1074. doi:10.1210/jcem.82.4.3862
- Wang C, Chan TK, Yeung RT, Coghlan JP, Scoggins BA, Stockigt JR. The effect of triamterene and sodium intake on renin, aldosterone, and erythrocyte sodium transport in Liddle’s syndrome. J Clin Endocrinol Metab 1981; 52(5):1027–1032. doi:10.1210/jcem-52-5-1027
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002; 970:134–144. pmid:12381548
- Saruta T, Suzuki H, Handa M, Igarashi Y, Kondo K, Senba S. Multiple factors contribute to the pathogenesis of hypertension in Cushing’s syndrome. J Clin Endocrinol Metab 1986; 62(2):275–279. doi:10.1210/jcem-62-2-275
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016; 4(7):569–576. doi:10.1016/S2213-8587(16)30005-5
- Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94(10):3857–3864. doi:10.1210/jc.2008-2766
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93(5):1526–1540. doi:10.1210/jc.2008-0125
- Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol 2015; 7:281–293. doi:10.2147/CLEP.S44336
- Tavares Bello C, van der Poest Clement E, Feelders R. Severe Cushing’s syndrome and bilateral pulmonary nodules: beyond ectopic ACTH. Endocrinol Diabetes Metab Case Rep 2017; pii:17–0100. doi:10.1530/EDM-17-0100
- Sathyakumar S, Paul TV, Asha HS, et al. Ectopic Cushing syndrome: a 10-year experience from a tertiary care center in southern India. Endocr Pract 2017; 23(8):907–914. doi:10.4158/EP161677.OR
In reply: Acid-base disturbances
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
A patient with altered mental status and an acid-base disturbance
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
A 78-year-old black woman with a history of osteoarthrosis and chronic diffuse joint pain presents with altered mental status and tachypnea, which began 3 hours earlier. She lives alone, and her family suspects she abuses both alcohol and her pain medications. She has not been eating well and has lost approximately 10 pounds over the past 3 months. Her analgesic regimen includes acetaminophen and acetaminophen-oxycodone.
In the emergency department her temperature is 98.6°F (37.0°C), pulse 100 beats per minute and regular, respiratory rate 22 per minute, and blood pressure 136/98 mm Hg. She is obtunded but has no focal neurologic defects or meningismus. She has no signs of heart failure (jugular venous distention, cardiomegaly, or gallops), and examination of the lungs and abdomen is unremarkable.
Suspecting that the patient may have taken too much oxycodone, the physician gives her naloxone, but her mental status does not improve. Results of chest radiography and cranial computed tomography are unremarkable. The physician’s initial impression is that the patient has “metabolic encephalopathy of unknown etiology.”
The patient’s laboratory values are shown in Table 1.
WHICH ACID-BASE DISORDER DOES SHE HAVE?
1. Which acid-base disorder does this patient have?
- Metabolic acidosis and respiratory alkalosis
- Metabolic acidosis and respiratory acidosis
- Metabolic acidosis with an elevated anion gap
- A triple disturbance: metabolic acidosis, respiratory acidosis, and metabolic alkalosis
A 5-step approach
Acid-base disorders can be diagnosed and characterized using a systematic approach known as the “Rules of 5” (Table 2)1:
1. Determine the arterial pH status.
2. Determine whether the primary process is respiratory, metabolic, or both.
3. Calculate the anion gap.
4. Check the degree of compensation (respiratory or metabolic).
5. If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased (ie, whether there is a delta gap).
Let us apply this approach to the patient described above.
1. What is her pH status?
An arterial pH less than 7.40 is acidemic, whereas a pH higher than 7.44 is alkalemic. (Acidemia and alkalemia refer to the abnormal laboratory value, while acidosis and alkalosis refer to the process causing the abnormal value—a subtle distinction, but worth keeping in mind.)
Caveat. A patient may have a significant acid-base disorder even if the pH is normal. Therefore, even if the pH is normal, one should verify that the partial pressure of carbon dioxide (Pco2), bicarbonate level, and anion gap are normal. If they are not, the patient may have a mixed acid-base disorder such as respiratory acidosis superimposed on metabolic alkalosis.
Our patient’s pH is 7.25, which is in the acidemic range.
2. Is her acidosis respiratory, metabolic, or both?
Respiratory acidosis and alkalosis affect the Pco2. The Pco2 is high in respiratory acidosis (due to failure to get rid of excess carbon dioxide), whereas it is low in respiratory alkalosis (due to loss of too much carbon dioxide through hyperventilation).
Metabolic acidosis and alkalosis, on the other hand, affect the serum bicarbonate level. In metabolic acidosis the bicarbonate level is low, whereas in metabolic alkalosis the bicarbonate level is high.
Moreover, in mixed respiratory and metabolic acidosis, the bicarbonate level can be low and the Pco2 can be high. In mixed metabolic and respiratory alkalosis, the bicarbonate level can be high and the Pco2 can be low (Table 2).
Our patient’s serum bicarbonate level is low at 16.0 mmol/L, indicating that the process is metabolic. Her Pco2 is also low (28 mm Hg), which reflects an appropriate response to compensate for the acidosis.
3. What is her anion gap?
Always calculate the anion gap, ie, the serum sodium concentration minus the serum chloride and serum bicarbonate concentrations. If the patient’s serum albumin level is low, for every 1 gram it is below normal, an additional 2.5 mmol/L should be added to the calculated anion gap. We consider an anion gap of 10 mmol/L or less as normal.
Caveats. The blood sample used to calculate the anion gap should be drawn close in time to the arterial blood gas sample.
Although the anion gap is an effective tool in assessing acid-base disorders, further investigation is warranted if clinical judgment suggests that an anion gap calculation is inconsistent with the patient’s circumstances.2
Our patient’s anion gap is elevated (21 mmol/L). Her serum albumin level is in the normal range, so her anion gap does not need to be adjusted.
4. Is the degree of compensation appropriate for the primary acid-base disturbance?
The kidneys compensate for the lungs, and vice versa. That is, in respiratory acidosis or alkalosis, the kidneys adjust the bicarbonate levels, and in metabolic acidosis, the lungs adjust the Pco2 (although in metabolic alkalosis, it is hard for patients to breathe less, especially if they are already hypoxic).
In metabolic acidosis, people compensate by breathing harder to get rid of more carbon dioxide. For every 1-mmol/L decrease in the bicarbonate level, the Pco2 should decrease by 1.3 mm Hg.
Compensation does not return pH to normal; rather, it mitigates the impact of an acid or alkali excess or deficit. If the pH is normalized with an underlying acid-base disturbance, there may be mixed acid-base processes rather than compensation.
Our patient’s bicarbonate level is 16 mmol/L, which is 9 mmol/L lower than normal (for acid-base calculations, we use 25 mmol/L as the nominal normal level). If she is compensating appropriately, her Pco2 should decline from 40 mm Hg (the nominal normal level) by about 11.7 mm Hg (9 × 1.3), to approximately 28.3 mm Hg. Her Pco2 is, indeed, 28 mm Hg, indicating that she is compensating adequately for her metabolic acidosis.
If we use Winter’s formula instead (Pco2 = [1.5 × the bicarbonate level] + 8 ± 2),3 the lowest calculated Pco2 would be 30 mm Hg, which is within 2 mm Hg of the Rules of 5 calculation. Other formulas for calculating compensation are available.3
This information rules out the first two answers to question 1, ie, metabolic acidosis with respiratory alkalosis or acidosis.
5. Is there a delta gap?
Although we know the patient has metabolic acidosis with an elevated anion gap, we have not ruled out the possibility that she may have a triple disturbance. For this reason we need to check her delta gap.
In metabolic acidosis with an elevated anion gap, as the bicarbonate level decreases, the anion gap should increase by the same amount. If the bicarbonate level decreases more than the anion gap increases, the additional decline is the result of a second process—an additional normal-anion-gap acidosis. If the bicarbonate level does not decrease as much as the anion gap increases, there is an additional metabolic alkalosis.
Our patient’s bicarbonate level decreased 9 mmol/L (from the nominal normal level of 25 to 16), and therefore her anion gap should have increased approximately the same amount—and it did. (A normal anion gap for problem-solving is 10, and this patient’s anion gap has increased to 21. A difference of ± 2 is insignificant.) This conclusion verifies that a triple acid-base disturbance is not present, so the last answer is incorrect.
So, the correct answer to the question posed above is metabolic acidosis with an elevated anion gap (that is, metabolic acidosis with appropriate respiratory compensation).
‘MUD PILES’: FINDING THE CAUSE OF ANION GAP METABOLIC ACIDOSIS
The possible causes of metabolic acidosis with an elevated anion gap (as in our patient) can be summarized in the mnemonic MUD PILES (methanol, uremia, diabetes, paraldehyde, isoniazid, lactate, ethylene glycol, and salicylates), which has been used for many years. Parts of it are no longer useful, but rather than discard it, we propose to update it (Table 3).
Methanol and ethylene glycol
We will address toxic ingestion of methanol and ethylene glycol (the “M” and “E” of MUD PILES) at the same time.
In cases of suspected ingestion of toxic substances such as these, it is useful to examine the osmol gap, ie, the difference between the calculated and the measured serum osmolality. Serum osmolality (in mOsm/kg) is calculated as the sodium concentration in mmol/L times 2, plus the glucose concentration in mg/dL divided by 18, plus the blood urea nitrogen concentration in mg/dL divided by 2.8 (Table 4). If the measured osmolality is higher than this calculated value, the difference may be due to solutes in the blood that should not be there such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products.
In our patient, ingestion of both methanol and ethylene glycol should be considered, since she lives alone and has been suspected of alcohol and opioid abuse. Her calculated osmol gap is 278 mOsm/kg. Her measured osmolality is 318 mOsm/kg (Table 1). The osmol gap is 40 mOsm/kg (normal is ≤ 10).4,5 Therefore, her osmol gap is elevated.
Identifying the specific substance the patient ingested that caused metabolic acidosis with anion gap may be difficult. Poisonings with these agents do not always increase the osmol gap.6 A high index of suspicion is essential. It is helpful to have the family search for any sources of ethylene glycol and methanol at home and initiate treatment early if an ingestion is suspected, using fomepizole (an alcohol dehydrogenase inhibitor) or parenteral ethanol and hemodialysis.7 Liquid chromatography identifies these two toxins, but results are not available emergently.
Diethylene glycol ingestion should also be considered.8 Since it is diagnosed and treated like ethylene glycol intoxication, it can be placed with the “E” of (di)ethylene glycol in the mnemonic.
Uremia
Renal failure can lead to metabolic acidosis.9 Our patient has no history of kidney disease, but her blood urea nitrogen and creatinine concentrations are above normal, and her estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula is 48 mL/min/1.73 m2—low, but not uremic.
Rhabdomyolysis (suspected by elevated creatine kinase values) should be considered in any patient with mental status changes, suspected toxic ingestion, and metabolic acidosis (see the “I” in MUD PILES below). Compartment syndromes with muscle necrosis may present in a subtle fashion. Therefore, renal failure from rhabdomyolysis may complicate this patient’s course later, and should be kept in mind.
Diabetes
The patient has no history of diabetes and has a normal blood glucose level. Blood testing did not reveal ketones. She is not taking metformin (alleged to cause lactic acidosis) or a sodium-glucose cotransporter 2 inhibitor (which have been associated with ketoacidosis).10
There is another, less common cause of ketoacidosis: alcohol.11 Although alcoholism is common, alcoholic ketoacidosis is uncommon, even in heavy drinkers. Ethyl alcohol causing metabolic acidosis is similar to metabolic acidosis with (di)ethylene glycol and methanol, and if suspected it should be treated empirically (first with thiamine, then dextrose and saline, and correcting other electrolyte disturbances such as hypokalemia and hypomagnesemia) before specific identification is made. Ketones (predominantly beta-hydroxybutyrate) may persist up to 2 weeks after alcohol ingestion has stopped.11 Ketosis in the setting of alcoholic ketoacidosis is frequently accompanied by other markers of alcohol target organ injury: elevated bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase levels. The term “ketohepatitis” has been suggested as an alternative to alcoholic ketosis.11
This patient did not have an elevated blood ethanol level, and her liver markers were otherwise normal.
THE NEW MUD PILES
2. Which of the following is (are) true? Regarding the remaining letters of the MUD PILES mnemonic:
- The “P” (paraldehyde) has been replaced by pyroglutamic acid (5-oxoproline) and propylene glycol.
- There are two isomers of lactate (dextro and levo), and consequently two clinical varieties of lactic acidosis.
- Isoniazid is no longer associated with metabolic acidosis with elevated anion gap.
- Salicylates can paradoxically be associated both with elevated and low anion gaps.
Isoniazid is still associated with metabolic acidosis with elevated anion gap, and so the third answer choice is false; the rest are true.
Paraldehyde, isoniazid, lactate
The “P,” “I,” and “L” (d-lactate) of the revamped MUD PILES acronym are less common than the others. They should be considered when the more typical causes of metabolic acidosis are not present, as in this patient.
UPDATING THE ‘P’ IN MUD PILES
Paraldehyde is rarely prescribed anymore. A PubMed search on December 21, 2015 applying the terms paraldehyde and metabolic acidosis yielded 17 results. Those specific to anion gap metabolic acidosis were from 1957 to 1986 (n = 9).12–20
Therefore, we can eliminate paraldehyde from the MUD PILES mnemonic and replace it with pyroglutamic acid and propylene glycol.
5-Oxoproline or pyroglutamic acid, a metabolite of acetaminophen
Acetaminophen depletes glutathione stores in acute overdoses, in patients with inborn errors of metabolism, and after chronic ingestion of excessive, frequent doses. Depletion of glutathione increases metabolic products, including pyroglutamic acid, which dissociates into hydrogen ions (leading to metabolic acidosis and an anion gap), and 5-oxoproline, (which can be detected in the urine).21,22
Risk factors for metabolic acidosis with acetaminophen ingestion include malnutrition, chronic alcoholism, liver disease, and female sex. In fact, most cases have been reported in females, and altered mental status has been common.
Metabolic acidosis with pyroglutamic acid can occur without elevated acetaminophen levels. Serum and urine levels of pyroglutamic acid may assist with diagnosis. Since identification of urine pyroglutamic acid usually requires outside laboratory assistance, a clinical diagnosis is often made initially and corroborated later by laboratory results. When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.
No controlled studies of treatment have been done. n-Acetylcysteine may be of benefit. Occasional patients have been dialyzed for removal of excess pyroglutamic acid.
Propylene glycol, a component of parenteral lorazepam
Lorazepam is a hydrophobic drug, so when it is given parenterally, it must be mixed with a suitable solvent. A typical formulation adds propylene glycol. In patients receiving high doses of lorazepam as relaxation therapy for acute respiratory distress syndrome in the intensive care unit, or as treatment of alcohol withdrawal, the propylene glycol component can precipitate anion gap metabolic acidosis.24,25
Although nearly one-half of the administered propylene glycol is excreted by the kidneys, the remaining substrate is metabolized by alcohol dehydrogenase into d,l-lactaldehyde, then converted into d- or l-lactate. l-Lactate can be metabolized, but d-lactate cannot and leads to anion gap metabolic acidosis. This is another toxic metabolic acidosis associated with an elevated osmol gap. An increasing osmol gap in the intensive care unit can serve as a surrogate marker of excessive propylene glycol administration.23
Isoniazid
Although it is uncommon, there are reports of isoniazid-induced anion gap metabolic acidosis,26 either due to overdoses, or less commonly, with normal dosing. Isoniazid should therefore remain in the mnemonic MUD PILES and may be suspected when metabolic acidosis is accompanied by seizures unresponsive to usual therapy. The seizures respond to pyridoxine.
The “I” should also be augmented by newer causes of metabolic acidosis associated with “ingestions.” Ecstasy, or 3,4-methylenedioxymethamphetamine, can cause metabolic acidosis and seizures. Ecstasy has been associated with rhabdomyolysis and uremia, also leading to anion gap metabolic acidosis.27 A newer class of abused substances, synthetic cathinones (“bath salts”), are associated with metabolic acidosis, compartment syndrome, and renal failure.28
Lactic acidosis
Lactic acidosis and metabolic acidosis can result from hypoperfusion (type A) or other causes (type B). Not all lactic acidosis is contingent on l-lactate, which humans can metabolize. Metabolic acidosis may be a consequence of d-lactate (mammals have no d-lactate dehydrogenase). d-Lactic acidosis as a result of short bowel syndrome has been known for more than a generation.29 However, d-lactic acidosis occurs in another new setting. The new “P” in MUD PILES, propylene glycol, can generate substantial amounts of d-lactate.29
d-lactic metabolic acidosis is always accompanied by neurologic manifestations (slurred speech, confusion, somnolence, ataxia, abusive behavior, and others).30 With short bowel syndrome, the neurologic manifestations occur after eating and clear later.30
Although our patient’s anion gap is more than 20 mmol/L, her blood level of lactate is not elevated, and she had no history to suggest short-bowel syndrome.
Salicylates
Salicylate overdose can cause a mixed acid-base disorder: metabolic acidosis with elevated anion gap and respiratory alkalosis.
Although our patient does not have respiratory alkalosis, an aspirin overdose must be considered. A salicylate level was ordered; it was negative.
Despite the typical association of salicylates with an elevated anion gap, they may also cause a negative anion gap.31 Chloride-sensing ion-specific electrodes contain a membrane permeable to chloride. Salicylates can increase the chloride permeability of these membranes, generating pseudohyperchloremia, and consequently, a negative anion gap.
WHAT ELSE MUST BE CONSIDERED?
3. In view of her anion gap metabolic acidosis, elevated osmol gap, and absence of diabetes, renal failure, or lactate excess, what are the remaining diagnoses to consider in this patient? (Choose all that are potential sources of metabolic acidosis and an increased anion gap.)
- Methanol, ethylene, or diethylene glycol
- Excessive, chronic acetaminophen ingestion
- Salicylate toxicity
- Alcoholic ketoacidosis
All of the above can potentially contribute to metabolic acidosis.
A search of the patient’s home did not reveal a source of methanol or either ethylene or diethylene glycol. Similarly, no aspirin was found, and the patient’s salicylate levels were not elevated. The patient’s laboratory work did not reveal increased ketones.
Since none of the common causes of metabolic acidosis were discovered, and since the patient had been taking acetaminophen, the diagnosis of excessive chronic acetaminophen ingestion was suspected pending laboratory verification. Identification of 5-oxoproline in the urine may take a week or more since the sample is usually sent to special laboratories. Acetaminophen levels in this patient were significantly elevated, as were urinary oxyproline levels, which returned later.
The patient was diagnosed with pyroglutamic acid metabolic acidosis. She was treated supportively and with n-acetylcysteine intravenously, although there have been no controlled studies of the efficacy of this drug. Seventy-two hours after admission, she had improved. Her acid-base status returned to normal.
GOLD MARK: ANOTHER WAY TO REMEMBER
Another mnemonic device for remembering the causes of metabolic acidosis with elevated anion gap is “GOLD MARK”: glycols (ethylene and propylene), oxoproline (instead of pyroglutamic acid from acetaminophen), l-lactate, d-lactate, methanol, aspirin, renal failure, and ketoacidosis).32
ACID-BASE DISORDERS IN DIFFERENT DISEASES
Diverse diseases cause distinctive acid-base abnormalities. Matching the appropriate acid-base abnormality with its associated disease may lead to more timely diagnosis and treatment:
Type 2 diabetes mellitus, for example, can lead to lactic acidosis, ketoacidosis, or type 4 renal tubular acidosis.33
Heart failure, although not typically framed in the context of acid-base physiology, can lead to elevated lactate, which is associated with a worse prognosis.34
Acquired immunodeficiency syndrome. Abacavir can cause normal anion gap metabolic acidosis.35,36
Cancer37,38 can be associated with proximal tubular renal tubular acidosis and lactic acidosis.
An expanding array of toxic ingestions
Metabolic acidosis may be the most prominent and potentially lethal clinical acid-base disturbance. When metabolic acidosis occurs in certain disease states—lactic acidosis with hypoperfusion or methanol ingestion with metabolic acidosis, for example—there is increased morbidity and mortality.
As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, pyroglutamic acid-oxoproline, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.
- Whittier WL, Rutecki GW. Primer on clinical acid-base problem solving. Dis Mon 2004; 50:122–162.
- Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2007; 2:162–174.
- Adrogué HJ, Madias NE. Secondary responses to altered acid-base status: the rules of engagement. J Am Soc Nephrol 2010; 21:920–923.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2012;12:1.
- Latus J, Kimmel M, Alscher MD, Braun N. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis—a single-centre experience. Clin Kidney J 2012; 5:120–123.
- Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol (Phila) 2015; 53:589–595.
- Ghannoum M, Hoffman RS, Mowry JB, Lavergne V. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial 2014; 27:395–401.
- Schep LJ, Slaughter RJ, Temple WA, Beasley DM. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009; 47:525–535.
- Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67:307–317.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Yokoyama A, Yokoyama T, Mizukami T, et al. Alcoholic ketosis: prevalence, determinants, and ketohepatitis in Japanese alcoholic men. Alcohol Alcohol 2014; 49:618–625.
- Hayward JN, Boshell BR. Paraldehyde intoxication with metabolic acidosis; report of two cases, experimental data and a critical review of the literature. Am J Med 1957; 23:965–976.
- Elkinton JR, Huth EJ, Clark JK, Barker ES, Seligson D. Renal tubular acidosis with organic aciduria during paraldehyde ingestion; six year study of an unusual case. Am J Med 1957; 23:977–986.
- Waterhouse C, Stern EA. Metabolic acidosis occurring during administration of paraldehyde. Am J Med 1957; 23:987–989.
- Beier LS, Pitts WH, Gonick HC. Metabolic acidosis occurring during paraldehyde intoxication. Ann Intern Med 1963; 58:155–158.
- Hiemcke T. Metabolic acidosis due to paraldehyde. Ned Tijdschr Geneeskd 1964; 108:2165–2167. Dutch.
- Gailitis RJ. Paraldehyde acidosis syndrome. IMJ III Med J 1966; 129:258–262.
- Gutman RA, Burnell JM. Paraldehyde acidosis. Am J Med 1967; 42:435–440.
- Hadden JW, Metzner RJ. Pseudoketosis and hyperacetaldehydemia in paraldehyde acidosis. Am J Med 1969; 47:642–647.
- Linter CM, Linter SP. Severe lactic acidosis following paraldehyde administration. Br J Psychiatry 1986; 149:650–651.
- Zand L, Muriithi A, Nelsen E, et al. Severe anion gap metabolic acidosis from acetaminophen use secondary to 5-oxoproline (pyroglutamic acid) accumulation. Am J Med Sci 2012; 344:501–504.
- Abkur TM, Mohammed W, Ali M, Casserly L. Acetaminophen-induced anion gap metabolic acidosis secondary to 5-oxoproline: a case report. J Med Case Rep 2014; 8:409.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy 2006; 26:23–33.
- Gokhale YA, Vaidya MS, Mehta AD, Rathod NN. Isoniazid toxicity presenting as status epilepticus and severe metabolic acidosis. J Assoc Physicians India 2009; 57:70–71.
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med 2003; 10:309–313.
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2–8.
- Jorens PG, Demey HE, Schepens PJ, et al. Unusual d-lactic acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol 2004; 42:163–169.
- Kang KP, Le S, Kang SK. d-Lactic acidosis in humans: review and update. Electrolyte Blood Press 2006; 4:53–56.
- Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis 2016; 67:143–150.
- Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st Century. Lancet 2008; 372:892.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Park JJ, Choi DJ, Yoon CH, et al; KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail 2015; 17:601–611.
- Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol 2016; 5:33–42.
- Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Diaz-Torres MA, Villareal-Velazquez HJ. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: a diagnostic challenge. Int J STD AIDS 2015; 26:1052–1054.
- Miltiadous G, Christidis D, Kalogirou M, Elisaf M. Causes and mechanisms of acid-base and electrolyte abnormalities in cancer. Eur J Intern Med 2008; 19:1–7.
- Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark 2015; 15:725–734.