User login
Assessing Refill Data Among Different Classes of Antidepressants (FULL)
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
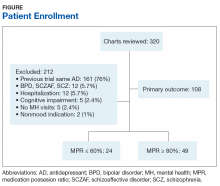
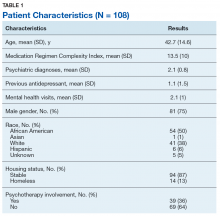
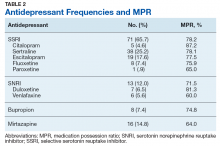
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
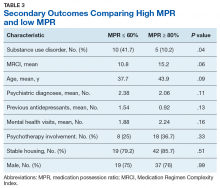
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.