User login
Endometriosis: From Identification to Management
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
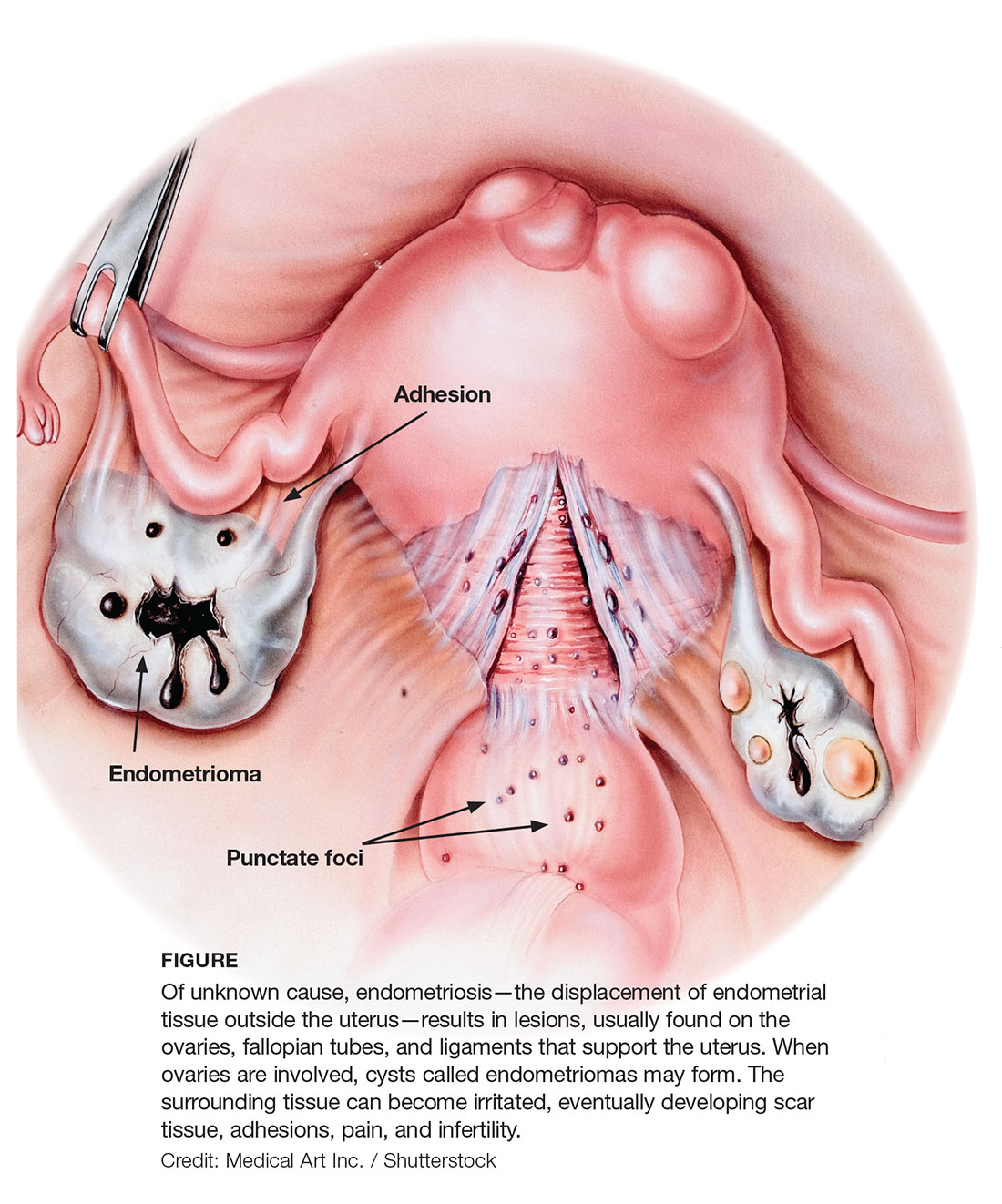
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
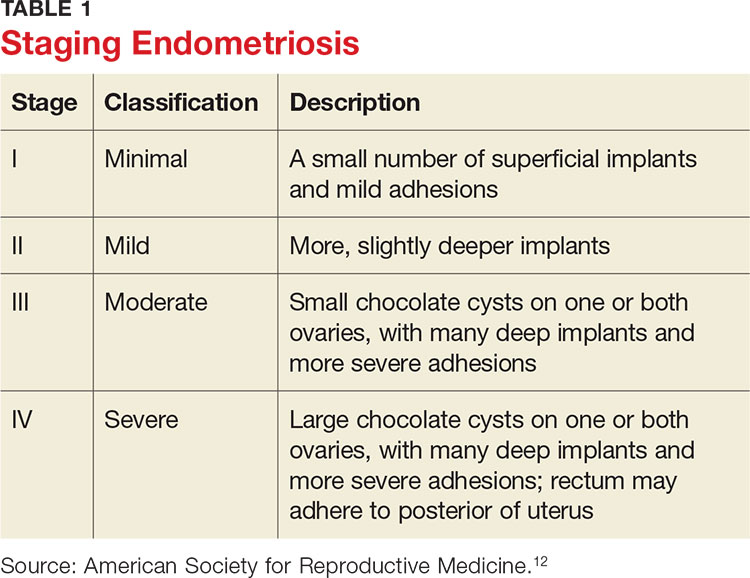
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
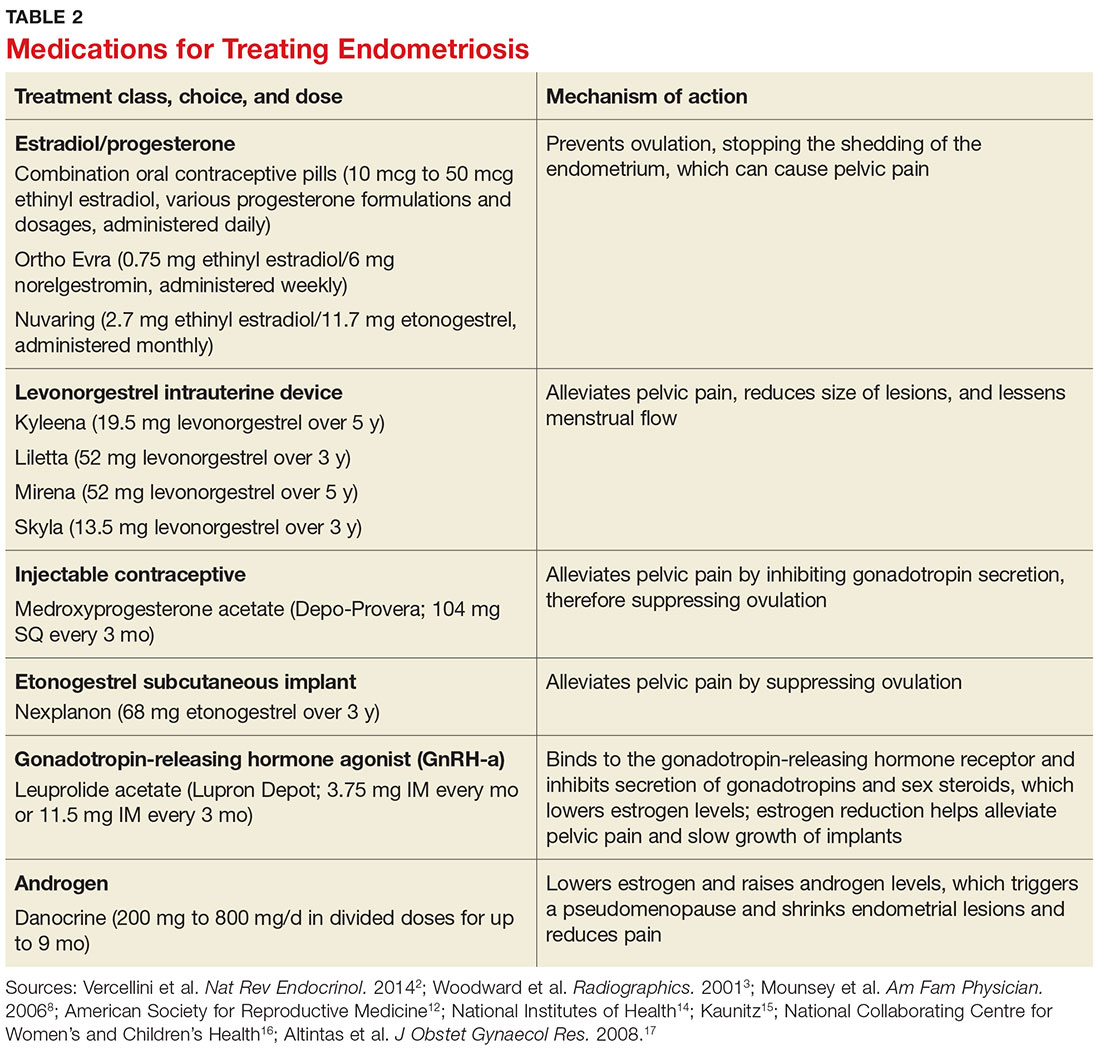
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
HPV Infection and Cervical Cancer Prevention
CE/CME No: CR-1309
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the central role that human papillomavirus (HPV) infection plays in the development of cervical intraepithelial neoplasia (CIN) and cervical cancer.
• Instruct patients on contributing cofactors of HPV infection and cervical cancer, including tobacco use, parity, use of oral contraceptives, co-infection with HIV, and immunosuppression.
• Describe the current recommendations for cervical cancer screening from professional societies, national health organizations, and federal agencies, including age-appropriate screening for cytology and high-risk HPV.
• Explain the timing and administration schedules of the currently available HPV vaccines and the patient populations for which the vaccines have been approved.
• Discuss the ablative and excisional procedures used to treat CIN and the treatment options for cervical cancer.
FACULTY
Heather P. Adams is an Assistant Professor/Clinical Coordinator in the Physician Assistant Program at Gannon University in Erie, Pennsylvania; in the program, Erica L. Carnright is a Physician Assistant student on clinical rotations.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Improved understanding of the central role of human papillomavirus (HPV) in cervical carcinogenesis has led to the development of vaccines and DNA testing for high-risk HPV subtypes. But age-appropriate cytologic screening remains the cornerstone in the prevention and early detection of cervical intraepithelial neoplasia (CIN) and cervical cancer. With prompt diagnosis and treatment of both CIN and early-stage cervical cancer, the prognosis for this disease is excellent.
Cervical cancer is the third most common cancer in women worldwide.1 More than 12,000 women are diagnosed with cervical cancer annually in the United States, with nearly 4,000 cervical cancer deaths reported each year.2-4 Globally, cervical cancer accounts for more than 529,000 new cancer cases and more than 275,000 cancer deaths in women annually, with more than 80% of these cases occurring in developing countries.1,5-7 Over the past 50 years, overall cervical cancer incidence and mortality have declined by more than 70% in the US, largely due to the development of the Papanicolaou screening test (Pap smear).7
Cervical cancer develops from precancerous, or neoplastic, cells of the cervix. Known as cervical intraepithelial neoplasia (CIN), these precancerous lesions are caused by infection with the human papillomavirus (HPV), the main etiologic factor in the development of cervical cancer. The progression from CIN to invasive cervical cancer is typically slow. Because of the long natural history of cervical cancer, screening and preventive measures, when appropriately implemented, can identify precancerous cells before they progress to cervical cancer.
EPIDEMIOLOGY AND RISK FACTORS
In 2013, approximately 12,340 new cases of cervical cancer will be diagnosed in the US, and 4,030 US women will die as a result of cervical cancer, according to National Cancer Institute estimates.8 Although the incidence rate of cervical cancer in the US has declined significantly, medically underserved populations remain disproportionately affected, with more than 60% of new cases occurring in underserved areas or underscreened populations.6 Epidemiologic studies have also shown higher incidence and mortality among minority populations.9 The incidence of cervical cancer is 30% higher among African American than white women, and the mortality rate is twice as high.9 The incidence of cervical cancer is highest among Hispanics.8
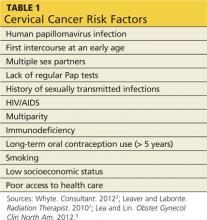
Although many risk factors contribute to the development of cervical cancer, HPV infection is the most important (see Table 12,7,9). HPV has been detected in up to 99% of all invasive cervical cancers, and its presence is necessary for oncogenesis to occur.10 HPV is the most common type of sexually transmitted infection (STI) in the US, with 75% to 80% of sexually active adults acquiring an HPV infection before age 50.11 It is estimated that a woman has a 10% lifetime risk for being infected with HPV, and this risk increases by 15% to 25% with each new sexual partner.7
Studies have shown that cigarette smoking contributes to the development of cervical cancer, with both previous and current smokers having a two- to threefold increased risk for high-grade CIN and cervical cancer, compared with never-smokers.5,9,12 Although the exact cause of this association has not been determined, carcinogens from cigarettes have been found within the cervical mucosa, which may cause alterations in the tissue.2,7
Long-term (≥ 5 years) use of oral contraceptive pills (OCP) by patients with an HPV cervical infection has been established as a risk factor for cervical cancer. It has been shown that the use of OCP causes an increase in HPV gene expression.5 In a study that compared two groups of women who tested positive for HPV DNA, the group that used OCP had a fourfold greater risk for cervical cancer than the group that did not use OCP.2,9,13
Parity, particularly multiparity, and HIV co-infection have also been implicated as risk factors for cervical cancer. The relationship between parity and cervical cancer likely results from the presence of the transformation zone on the ectocervix and increased squamous metaplasia during pregnancy, both of which make cervical tissue more vulnerable to HPV infection.14 HIV co-infection increases the risk for cervical cancer secondary to its immunosuppressive effects; women with both HIV and high-risk HPV infections are four times more likely to develop cervical cancer.15
On the next page: Cervical anatomy >>
CERVICAL ANATOMY
The cervix is the narrow, fibromuscular neck that makes up the lower portion of the uterus. The endocervix is the cervical canal, and the ectocervix extends inferiorly into the vagina.7 The internal os is the opening of the cervix into the uterus, and the external os is the opening of the cervix into the vagina. The endocervix is lined with glandular columnar epithelium. This tissue can extend beyond the external os onto the ectocervix, and when this occurs it is known as cervical ectopy.16 The surface of the cervix lying between the glandular tissue and the vaginal wall is comprised of stratified nonkeratinizing squamous epithelium.7 The squamocolumnar junction (SCJ) is defined as the location where the squamous and glandular cells meet.17 Adjacent to the SCJ is the transformation zone, an area vulnerable to HPV infection where glandular cells are actively undergoing squamous metaplasia.17
The location of the SCJ varies depending on age and hormonal changes. In prepubertal females, the SCJ is close to the external os, but in women of reproductive age, the SCJ moves away from the external os onto the surface of the ectocervix. This change in the location of the SCJ occurs due to increased estrogen levels following menarche, which cause the endocervical canal to elongate.7,17 A satisfactory Pap smear for cervical cytology includes cells from the transformation zone, as well as the ectocervix and endocervix.
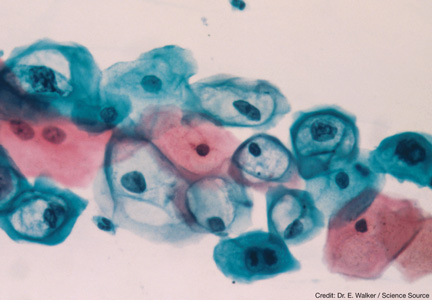
HUMAN PAPILLOMAVIRUS
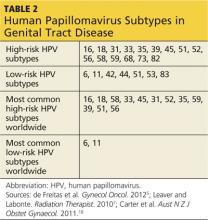
The human papillomavirus is a nonenveloped, double-stranded DNA virus that is known to cause abnormalities in skin cells. This virus is predominantly spread through sexual contact via skin-to-skin transmission. There are more than 100 known subtypes in the HPV family, of which 40 subtypes have been recognized to cause genital tract disease. Of these 40 subtypes, 15 have been identified to be oncogenic.9,18,19
HPV subtypes are separated into low-risk and high-risk categories (see Table 25,7,18). The low-risk subtypes cause either no cellular change, low-grade intraepithelial neoplasia, or condyloma. The high-risk subtypes are associated with the development of CIN and cancer. Of the 15 high-risk subtypes, HPV-16 and HPV-18 are the most oncogenic, followed by HPV-31 and HPV-45. HPV-16 is implicated in up to 70% of cervical cancers, HPV-18 in up to 20%, and HPV-31 and HPV-45 in up to 10%.5,9,20 Of the low-risk HPV subtypes, HPV-6 and HPV-11 cause 90% of condyloma infections.16,21
After HPV infection is contracted, the virus migrates into the squamous epithelial mucosa of the cervix, where it is capable of altering cells and causing the development of precancerous properties. This typically happens within the transformation zone, which is vulnerable to HPV infection.17 Depending on the state of the host’s immune system, many of these HPV infections clear without intervention. It is estimated that approximately 70% of HPV infections resolve spontaneously within one year, and 90% resolve within two years.5 HPV infections that persist beyond two years are more likely to lead to the development of CIN and, ultimately, cervical cancer, if left untreated.22 There is an association between genetic amplification (extra copies) of gene 3q26 and progression of CIN to cervical cancer, but ultimately a high-risk HPV subtype must be involved for cervical cancer to develop.23
On the next page: Clinical manifestations, screening, and prevention >>
CLINICAL MANIFESTATIONS
Due to the widespread use of cervical cytology, many cases of CIN and cervical cancer are diagnosed well before symptoms develop. In the early stages of cervical cancer, most women are asymptomatic, although some women may present with a watery, blood-tinged, or malodorous vaginal discharge.7 Any type of abnormal bleeding, whether it is between menstrual cycles, postcoital, or postmenopause, warrants an evaluation for pathologic processes, including but not limited to cervical cancer. Patients with late-stage cervical cancer may present with complaints of pelvic pain and painful intercourse due to the enlargement of a cervical mass. Vaginal discharge may change in consistency from watery to purulent and become foul-smelling due to cervical tissue necrosis.7,9,22 In some cases of advanced invasive cervical cancer, patients will experience hematuria or rectal bleeding secondary to tumor invasion through the bladder or rectal wall.9 Other nonspecific signs and symptoms of cervical cancer include unexplained weight loss accompanied by nausea or vomiting, as well as loss of appetite.7,9
SCREENING AND PREVENTION
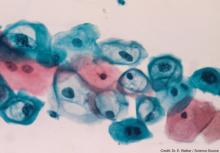
Widespread use of the Pap test since the 1950s has led to marked reductions in the incidence of cervical cancer. The Pap test enables clinicians to screen for and detect CIN. More recently, liquid-based cytology has been utilized for the same purpose. The Pap smear involves placing the sample cells directly onto a microscopic slide. With liquid-based cytology, the sample is placed into a vial,2,7 where a portion is processed in preservative liquid under light microscopy for review by a cytologist.7 The remainder of the cell sample can be tested for HPV and STIs.
DNA testing for high-risk HPV subtypes is available and has been incorporated into the cervical cancer screening guidelines. The guidelines recommend HPV DNA testing as a co-test to be performed with cytology in women older than 30.2,24 The recommendation for HPV DNA testing in this age-group reflects greater concern that older women’s immune systems will not clear the virus, leaving these women at higher risk for developing CIN and cancer.25,26 HPV-16 and HPV-18 genotyping is available and is recommended in women 30 or older whose co-testing reveals normal cytology in the presence of a high-risk HPV subtype.27
Additionally, HPV DNA testing is used to triage cytology that reveals atypical squamous cells of undetermined significance. HPV DNA testing is not performed routinely in younger women because of the high likelihood that their immune systems will clear the virus. Genetic testing for the 3q26 gene amplification is available but has not yet been incorporated into the screening guidelines.27
Screening Guidelines
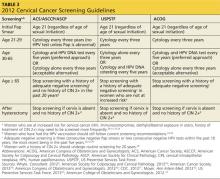
In September 2012, the American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology developed a new set of cervical cancer screening guidelines.28 The US Preventive Services Task Force (USPSTF) and American College of Obstetricians and Gynecologists (ACOG) have also created standard recommendations for cervical cancer screening (see Table 32,28-34). Although ACOG’s standards have varied from those of other organizations in the past, their 2012 guidelines now align closely.
All these organizations recommend that cervical screening begin at age 21 for all women, regardless of the age of sexual initiation.31 Many young women who acquire an HPV infection will clear the infection within the first one to two years. Eliminating screening of women younger than 21 will help to avoid unnecessary biopsies and invasive treatment.29-32
All the guidelines include general recommendations for screening; however, each organization has made exceptions for patients at higher risk for developing cervical cancer.2,28-34 ASCCP has also developed algorithms for management of the multitude of abnormal cervical cancer screening results. These algorithms are available through the ASCCP Web site (www.asccp.org).
Vaccinations
HPV vaccines developed in recent years are now helping to prevent HPV infections from occurring. Currently, the FDA has approved the use of two HPV vaccines: a quadrivalent recombinant HPV vaccine (Gardasil) in 2006 and a bivalent HPV vaccine in 2009 (Cervarix).2 The quadrivalent vaccine was approved for girls and women ages 9 to 26 and protects against HPV subtypes 6 and 11—two low-risk HPV subtypes that can cause genital warts—and subtypes 16 and 18—two high-risk HPV subtypes that can cause cervical cancer. This vaccine is given in a three-dose series at months 0, 2, and 6, and should be initiated before women become sexually active for maximum effectiveness.22,35 The quadrivalent vaccine received additional FDA approval for administration in boys and men ages 9 to 26 in 2010.36
The bivalent vaccine is given in a three-dose series as well and is approved for girls and women ages 9 to 25.37 This vaccine offers protection against high-risk HPV subtypes 16 and 18.38
Recent trials have shown that both the quadrivalent and bivalent vaccines are more than 90% effective in preventing the development of precancerous cells from HPV subtypes 16 and 18.2 Although these vaccines provide protection against the two most oncogenic HPV subtypes, routine cytologic testing is still recommended in patients who receive the vaccines because they do not protect against all high-risk subtypes.2,35,38
On the next page: Diagnosis and staging >>
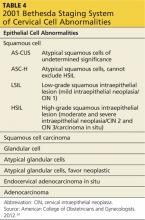
DIAGNOSIS AND STAGING
The Bethesda staging system provides uniform terminology to classify all abnormal Pap smears and cytology reports (see Table 434). Patients with abnormal cytology typically undergo a colposcopic examination to further evaluate the abnormality. During a colposcopy, the clinician examines the cervix with the use of a lighted microscope, known as a colposcope, which magnifies the cells of the cervix and allows for localized sampling of abnormal-appearing areas through punch biopsy.7,9 The area of focus is the transformation zone because of its known vulnerability to HPV infections. A colposcopy is considered inadequate if the SCJ, and therefore the transformation zone, is not fully visualized.
Acetic acid is applied to the cervix during colposcopy, and any dysplastic cells present take up the acid and turn white, a process known as acetowhitening. Indications for a punch biopsy include acetowhitening, leukoplakia, and abnormal vasculature marked by punctation, bizarre-appearing vessels, or the appearance of a mosaic pattern.16 Indications for sampling of the endocervical canal through endocervical curettage include inadequate colposcopy, presence of a lesion that extends beyond the view of the colposcope, or atypical glandular cells on cytology.16 The biopsy results help to determine if a diagnostic conization is needed for further evaluation of the lesion.16
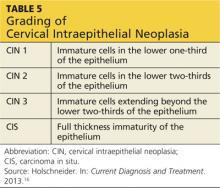
After a cervical biopsy is performed, the abnormal cells may be classified as CIN 1, 2, or 3 or carcinoma in situ (see Table 516). These stages of intraepithelial neoplasia are determined by the depth to which abnormal, immature cells have invaded the cervical epithelium.16 CIN 2 and 3 are more likely to develop into cervical cancer if they are not properly treated.5,16,17 The majority of CIN 1 lesions do not progress into cancer and typically resolve on their own or are cleared by the patient’s immune system. While most CIN 1 lesions are caused by high-risk HPV, these may be less oncogenic subtypes.7,17
The two most common histologic subtypes of cervical cancer are squamous cell carcinoma and adenocarcinoma. Squamous cell carcinoma comprises more than 70% of cervical cancers, while adenocarcinoma makes up approximately 25%.39 Neuroendocrine, small cell, and mixed-cell carcinomas make up the remainder of cervical cancers.9 Squamous cell carcinoma arises from the squamocolumnar junction or ectocervix, and adenocarcinoma tends to arise from the glandular cells of the endocervix.7,9,17 Studies show that adenocarcinoma is slowly becoming more prevalent in the US than squamous cell carcinoma.9
Once a diagnosis of cervical cancer has been established, additional testing and procedures are performed to rule out lymph node and organ involvement.9,16 The International Federation of Gynecology and Oncology system is then used to clinically stage the cervical cancer40 (see Table 67,40,41). This staging system, updated in 2009, is based solely on clinical examination findings. Once cervical cancer is staged, measures are taken to assess which treatment option is most appropriate.
On the next page: Treatment modalities >>
TREATMENT MODALITIES
Treatment strategies for CIN correspond to the degree of neoplasia and adequacy of colposcopy, and tend to vary among clinicians. For invasive cervical cancer, findings such as lymph node dissemination and adjacent organ involvement are key factors in determining which therapy will be selected.16
Cervical Intraepithelial Neoplasia
The treatment options for CIN can be divided into two categories, ablative and excisional. These techniques may be used only when invasive cervical cancer has been excluded.16,42 Cryotherapy and laser ablation are two common ablative techniques. One disadvantage of ablative techniques is that abnormal tissue is destroyed during the procedure, making it impossible for a tissue specimen to be collected and sent for additional evaluation.42,43 Cold knife conization, laser cone excision, and loop electrosurgical excision procedure (LEEP) are all excisional treatments that allow tissue to be collected and further evaluated.44 Although all these procedures can be used for any stage of CIN, excisional procedures are preferred for CIN 2 and CIN 3 lesions because moderate and severe intraepithelial neoplasias have a higher incidence of undetected microinvasive disease within the endocervical canal. Ablative therapy is generally reserved for treating low-grade CIN or small, focal high-grade lesions.42
Ablative techniques. Cryotherapy was the first outpatient treatment developed for CIN and is still widely used due to its ease, low cost, and low complication rate.44 It is an in-office procedure that utilizes nitrous oxide and carbon dioxide to freeze and ablate abnormal cells on the ectocervix. The procedure involves a freeze-thaw-freeze technique that has improved the efficacy of this treatment.16 The main side effects of cryotherapy are mild cramping and copious watery discharge that can last for up to 4 weeks postprocedure.42,45
Laser ablation is not used as commonly as cryosurgery, but is a viable option for women with large CIN lesions or women unwilling to undergo a LEEP. This procedure is performed under either local or general anesthesia and involves the use of a carbon dioxide laser under colposcopic guidance to ablate the transformation zone of the cervix.45 Laser ablation completely destroys the lesion while causing minimal damage to the surrounding, unaffected tissue. After treatment, patients may experience vaginal discharge for approximately 1 to 2 weeks.44,45 Overall, this technique is very expensive and requires a substantial amount of training to achieve maximum effectiveness.44
Excisional techniques. The LEEP is the procedure of choice for treating moderate and high-grade lesions. LEEP uses a wire loop electrode to excise the transformation zone of the cervix. Acetic acid or an iodine solution known as Lugol’s solution is applied to the cervix to delineate the margins of the lesion.42 The majority of these procedures can be done under local anesthesia, but on occasion sedation may be required.44 Complications, although rare, include post-treatment bleeding, infection, cervical stenosis, and cervical incompetence.45
Excisional conization is a procedure that can be performed with a scalpel (cold knife conization) or with a laser (laser cone excision).45 This procedure involves the removal of a cone-shaped portion of the cervix. Following the conization, endocervical curettage may be performed to acquire tissue samples and assess the endocervical canal. Also known as a cone biopsy, the procedure is typically performed under sedation. The types of complications associated with excisional conization are the same as those for LEEP, but the complication rate is higher when cone biopsy is performed.16,45
CERVICAL CANCER
Treatment methods for cervical cancer can be classified according to one of three disease stages: early-stage (IA2-IIA2), locally advanced (IIB-IVA), and advanced disease (IVB). Common treatment options for cervical cancer include radical hysterectomy with pelvic lymphadenectomy, radiation, and chemotherapy.9,17 Patients diagnosed with early-stage disease typically undergo a radical hysterectomy with pelvic lymphadenectomy. Cervical conization is an alternative option for young women who want to preserve fertility and wish to avoid a hysterectomy.45 Women who undergo surgery and are found to have more extensive disease or parametrial invasion are offered adjuvant radiation therapy.9
Patients diagnosed with locally advanced cancer are typically treated with radiation, chemotherapy, or both.17,45 The combination of radiation and chemotherapy in cervical cancer has been shown to increase survival rates in late-stage disease.7 Those diagnosed with advanced or disseminated disease are treated with palliative radiation. Overall, the five-year survival rates for patients diagnosed with early-stage cervical cancer who receive appropriate treatment have proven to be excellent7 (see Table 77,40,46).
On the next page: Conclusion >>
CONCLUSION
The incidence and prevalence of cervical cancer continue to decline in the US; the majority of cases that arise are due to the lack of knowledge and availability of screening services in underserved areas. Increasing awareness and developing clinics for these under-screened populations would most likely contribute to a further decline in the incidence of CIN and cervical cancer.
Once diagnosed with an HPV infection, women should be informed that the body generally clears these infections within 1 to 2 years. Persistence of a high-risk HPV infection greatly increases the risk for neoplastic tissue transformation. These women need to be followed diligently so that treatment can be implemented at the first signs of a CIN 2 or higher grade lesion. Ultimately, implementing adequate screening techniques allows for early detection of CIN and prevention of cervical cancer.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69-90.
2. Whyte J. HPV and cervical cancer: latest developments. Consultant. 2012;52:555-560.
3. CDC. Cervical cancer statistics. www.cdc.gov/cancer/cervical/statistics/. Accessed July 23, 2013.
4. National Cancer Institute. Human papillomavirus (HPV) vaccines. www.cancer.gov/cancertopics/factsheet/prevention/HPV-vaccine. Accessed July 23, 2013.
5. de Freitas AC, Gurgel AP, Chagas BS, et al. Susceptibility to cervical cancer: an overview. Gynecol Oncol. 2012;126:304-311.
6. Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531-2542.
7. Leaver D, Labonte G. HPV and cervical cancer. Radiation Therapist. 2010;19:27-45.
8. National Cancer Institute. SEER stat facts sheets: Cervix uteri cancer. http://seer.cancer.gov/statfacts/html/cervix.html#incidence-mortality. Accessed July 5, 2013.
9. Lea JS, Lin KY. Cervical cancer. Obstet Gynecol Clin North Am. 2012;39:233-253.
10. McKeever AE. Cervical cancer risk among college-age women: a review of the literature. SGNO J. 2010;20:6-12.
11. Frumovitz M. Invasive cervical cancer: epidemiology, risk factors, clinical manifestations, and diagnosis. UpToDate. Goff B, Falk SJ, eds. UpToDate, Waltham. MA, 2013. www.uptodate.com/contents/invasive-cervical-cancer-epidemiology-risk-factors-clinical-manifestations-and-diagnosis?detectedLanguage=en&source=search_result&search=Lifetime+prevalence+of+HPV+infection&selectedTitle=10%7E150&provider=noProvider. Accessed July 23, 2013.
12. Collins S, Rollason TP, Young LS, Woodman CB. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: a longitudinal study. Eur J Cancer. 2010;46:405-411.
13. Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085-1092.
14. Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359(9312):1093-1101.
15. Kalra S. Studies examine relationship between HIV and cervical cancer in women. The AIDS Beacon. 2010. www.aidsbeacon.com/news/2010/07/23/studies-examine-relationship-between-hiv-and-cervical-cancer-in-women-aids-2010/. Accessed July 6, 2013.
16. Holschneider CH. Premalignant and malignant disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics and Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:807-808, 811-816, 820.
17. Rajaram S, Chitrathara K, Maheshwari A. Cervical Cancer: Contemporary Management. 1st ed. New Dehli, India: Jaypee Brothers Medical Publishers; 2012.
18. Carter JR, Ding Z, Rose BR. HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol. 2011;51:103-108.
19. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5-10.
20. Khachikyan I, Stratton P. Benign disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics & Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:650.
21. CDC. 2010 sexually transmitted disease surveillance. www.cdc.gov/std/stats10/other.htm. Accessed July 23, 2013.
22. Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82:1209-1214.
23. Verri A, Jalali GR, Cecchini G, et al. Significant progression of uterine cervical epithelial lesion accompanied by marked increase in 3q26 gene amplification. Lab Med. 2010;42:134-136.
24. Schwaiger C, Aruda M, LaCoursiere S, Rubin R. Current guidelines for cervical cancer screening. J Am Acad Nurse Prac. 2012;24:417-424.
25. CDC. Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older. www.cdc.gov/cancer/hpv/basic_info/screening/cervical_screening.htm. Accessed July 5, 2013.
26. Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103: 368-383.
27. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updates consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1-S27.
28. American Society for Colposcopy and Cervical Pathology. Cervical cancer screening recommendations, 2012. www.asccp.org/Portals/9/docs/pdfs/Practice%20Management/ASCCP_Cervical_Cancer_Screening_Recommendations.pdf#zoom=80. Accessed July 6, 2013.
29. American Cancer Society. New screening guidelines for cervical cancer. March 14, 2012. www.cancer.org/cancer/news/news/new-screening-guidelines-for-cervical-cancer. Accessed July 23, 2013.
30. American Congress of Obstetricians and Gynecologists. USPSTF updated cervical cancer screening. www.acog.org/~/media/Districts/District%20II/PDFs/USPSTF_Cervical_Ca_Screening_Guidelines.pdf?dmc=1&ts=20130723T1510069242. Accessed July 23, 2013.
31. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed July 5, 2013.
32. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
33. US Preventive Services Task Force. Screening for cervical cancer. Current recommendation. www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm#clinical. Accessed July 6, 2013.
34. American College of Obstetricians and Gynecologists; Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120:1222-1238.
35. Warman J. Cervical cancer screening in young women: saving lives with prevention and detection. Oncol Nurs Forum. 2010;37:33-38.
36. Gardasil [package insert]. Whitehouse Station, NJ: Merck & Co; 2011.
37. Cervarix [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
38. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
39. Morrison RS, Moody R, Shelton M. Pap smear rates: predictor of cervical cancer mortality disparity? Online J Rural Nurs Health Care. 2010;10:21-27.
40. National Cancer Institute. Cervical cancer treatment. www.cancer.gov/cancertopics/pdq/treatment/cervical/HealthProfessional/page1/AllPages#2.
41. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-104.
42. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99. management of abnormal cervical cytology and histology. J Obstet Gynecol. 2008;112:1419-1444.
43. Bowring J, Tulloch I, Phadnis SV, et al. Secondary excision for cervical intraepithelial neoplasia: an evaluation of two treatment methods. J Obstet Gynaecol. 2010;30:511-514.
44. Jhingran A. Neoplasms of the cervix. In: Hong KW, Bast RC Jr, Hait WN, et al, eds. Holland-Frei Cancer Medicine. 8th ed. Ontario, Canada: BC Decker; 2010:1304-1312.
45. Kim SH. Preinvasive disease of the lower genital tract. In: Chu CS, Rubin SC, eds. Manual of Gynecologic Oncology. 1st ed. Hackensack, NJ: World Scientific Publishing Company; 2011:80-87.
46. American Cancer Society. Cervical cancer key statistics. http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statistics. Accessed July 5, 2013.
CE/CME No: CR-1309
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the central role that human papillomavirus (HPV) infection plays in the development of cervical intraepithelial neoplasia (CIN) and cervical cancer.
• Instruct patients on contributing cofactors of HPV infection and cervical cancer, including tobacco use, parity, use of oral contraceptives, co-infection with HIV, and immunosuppression.
• Describe the current recommendations for cervical cancer screening from professional societies, national health organizations, and federal agencies, including age-appropriate screening for cytology and high-risk HPV.
• Explain the timing and administration schedules of the currently available HPV vaccines and the patient populations for which the vaccines have been approved.
• Discuss the ablative and excisional procedures used to treat CIN and the treatment options for cervical cancer.
FACULTY
Heather P. Adams is an Assistant Professor/Clinical Coordinator in the Physician Assistant Program at Gannon University in Erie, Pennsylvania; in the program, Erica L. Carnright is a Physician Assistant student on clinical rotations.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Improved understanding of the central role of human papillomavirus (HPV) in cervical carcinogenesis has led to the development of vaccines and DNA testing for high-risk HPV subtypes. But age-appropriate cytologic screening remains the cornerstone in the prevention and early detection of cervical intraepithelial neoplasia (CIN) and cervical cancer. With prompt diagnosis and treatment of both CIN and early-stage cervical cancer, the prognosis for this disease is excellent.
Cervical cancer is the third most common cancer in women worldwide.1 More than 12,000 women are diagnosed with cervical cancer annually in the United States, with nearly 4,000 cervical cancer deaths reported each year.2-4 Globally, cervical cancer accounts for more than 529,000 new cancer cases and more than 275,000 cancer deaths in women annually, with more than 80% of these cases occurring in developing countries.1,5-7 Over the past 50 years, overall cervical cancer incidence and mortality have declined by more than 70% in the US, largely due to the development of the Papanicolaou screening test (Pap smear).7
Cervical cancer develops from precancerous, or neoplastic, cells of the cervix. Known as cervical intraepithelial neoplasia (CIN), these precancerous lesions are caused by infection with the human papillomavirus (HPV), the main etiologic factor in the development of cervical cancer. The progression from CIN to invasive cervical cancer is typically slow. Because of the long natural history of cervical cancer, screening and preventive measures, when appropriately implemented, can identify precancerous cells before they progress to cervical cancer.
EPIDEMIOLOGY AND RISK FACTORS
In 2013, approximately 12,340 new cases of cervical cancer will be diagnosed in the US, and 4,030 US women will die as a result of cervical cancer, according to National Cancer Institute estimates.8 Although the incidence rate of cervical cancer in the US has declined significantly, medically underserved populations remain disproportionately affected, with more than 60% of new cases occurring in underserved areas or underscreened populations.6 Epidemiologic studies have also shown higher incidence and mortality among minority populations.9 The incidence of cervical cancer is 30% higher among African American than white women, and the mortality rate is twice as high.9 The incidence of cervical cancer is highest among Hispanics.8
Although many risk factors contribute to the development of cervical cancer, HPV infection is the most important (see Table 12,7,9). HPV has been detected in up to 99% of all invasive cervical cancers, and its presence is necessary for oncogenesis to occur.10 HPV is the most common type of sexually transmitted infection (STI) in the US, with 75% to 80% of sexually active adults acquiring an HPV infection before age 50.11 It is estimated that a woman has a 10% lifetime risk for being infected with HPV, and this risk increases by 15% to 25% with each new sexual partner.7
Studies have shown that cigarette smoking contributes to the development of cervical cancer, with both previous and current smokers having a two- to threefold increased risk for high-grade CIN and cervical cancer, compared with never-smokers.5,9,12 Although the exact cause of this association has not been determined, carcinogens from cigarettes have been found within the cervical mucosa, which may cause alterations in the tissue.2,7
Long-term (≥ 5 years) use of oral contraceptive pills (OCP) by patients with an HPV cervical infection has been established as a risk factor for cervical cancer. It has been shown that the use of OCP causes an increase in HPV gene expression.5 In a study that compared two groups of women who tested positive for HPV DNA, the group that used OCP had a fourfold greater risk for cervical cancer than the group that did not use OCP.2,9,13
Parity, particularly multiparity, and HIV co-infection have also been implicated as risk factors for cervical cancer. The relationship between parity and cervical cancer likely results from the presence of the transformation zone on the ectocervix and increased squamous metaplasia during pregnancy, both of which make cervical tissue more vulnerable to HPV infection.14 HIV co-infection increases the risk for cervical cancer secondary to its immunosuppressive effects; women with both HIV and high-risk HPV infections are four times more likely to develop cervical cancer.15
On the next page: Cervical anatomy >>
CERVICAL ANATOMY
The cervix is the narrow, fibromuscular neck that makes up the lower portion of the uterus. The endocervix is the cervical canal, and the ectocervix extends inferiorly into the vagina.7 The internal os is the opening of the cervix into the uterus, and the external os is the opening of the cervix into the vagina. The endocervix is lined with glandular columnar epithelium. This tissue can extend beyond the external os onto the ectocervix, and when this occurs it is known as cervical ectopy.16 The surface of the cervix lying between the glandular tissue and the vaginal wall is comprised of stratified nonkeratinizing squamous epithelium.7 The squamocolumnar junction (SCJ) is defined as the location where the squamous and glandular cells meet.17 Adjacent to the SCJ is the transformation zone, an area vulnerable to HPV infection where glandular cells are actively undergoing squamous metaplasia.17
The location of the SCJ varies depending on age and hormonal changes. In prepubertal females, the SCJ is close to the external os, but in women of reproductive age, the SCJ moves away from the external os onto the surface of the ectocervix. This change in the location of the SCJ occurs due to increased estrogen levels following menarche, which cause the endocervical canal to elongate.7,17 A satisfactory Pap smear for cervical cytology includes cells from the transformation zone, as well as the ectocervix and endocervix.
HUMAN PAPILLOMAVIRUS
The human papillomavirus is a nonenveloped, double-stranded DNA virus that is known to cause abnormalities in skin cells. This virus is predominantly spread through sexual contact via skin-to-skin transmission. There are more than 100 known subtypes in the HPV family, of which 40 subtypes have been recognized to cause genital tract disease. Of these 40 subtypes, 15 have been identified to be oncogenic.9,18,19
HPV subtypes are separated into low-risk and high-risk categories (see Table 25,7,18). The low-risk subtypes cause either no cellular change, low-grade intraepithelial neoplasia, or condyloma. The high-risk subtypes are associated with the development of CIN and cancer. Of the 15 high-risk subtypes, HPV-16 and HPV-18 are the most oncogenic, followed by HPV-31 and HPV-45. HPV-16 is implicated in up to 70% of cervical cancers, HPV-18 in up to 20%, and HPV-31 and HPV-45 in up to 10%.5,9,20 Of the low-risk HPV subtypes, HPV-6 and HPV-11 cause 90% of condyloma infections.16,21
After HPV infection is contracted, the virus migrates into the squamous epithelial mucosa of the cervix, where it is capable of altering cells and causing the development of precancerous properties. This typically happens within the transformation zone, which is vulnerable to HPV infection.17 Depending on the state of the host’s immune system, many of these HPV infections clear without intervention. It is estimated that approximately 70% of HPV infections resolve spontaneously within one year, and 90% resolve within two years.5 HPV infections that persist beyond two years are more likely to lead to the development of CIN and, ultimately, cervical cancer, if left untreated.22 There is an association between genetic amplification (extra copies) of gene 3q26 and progression of CIN to cervical cancer, but ultimately a high-risk HPV subtype must be involved for cervical cancer to develop.23
On the next page: Clinical manifestations, screening, and prevention >>
CLINICAL MANIFESTATIONS
Due to the widespread use of cervical cytology, many cases of CIN and cervical cancer are diagnosed well before symptoms develop. In the early stages of cervical cancer, most women are asymptomatic, although some women may present with a watery, blood-tinged, or malodorous vaginal discharge.7 Any type of abnormal bleeding, whether it is between menstrual cycles, postcoital, or postmenopause, warrants an evaluation for pathologic processes, including but not limited to cervical cancer. Patients with late-stage cervical cancer may present with complaints of pelvic pain and painful intercourse due to the enlargement of a cervical mass. Vaginal discharge may change in consistency from watery to purulent and become foul-smelling due to cervical tissue necrosis.7,9,22 In some cases of advanced invasive cervical cancer, patients will experience hematuria or rectal bleeding secondary to tumor invasion through the bladder or rectal wall.9 Other nonspecific signs and symptoms of cervical cancer include unexplained weight loss accompanied by nausea or vomiting, as well as loss of appetite.7,9
SCREENING AND PREVENTION
Widespread use of the Pap test since the 1950s has led to marked reductions in the incidence of cervical cancer. The Pap test enables clinicians to screen for and detect CIN. More recently, liquid-based cytology has been utilized for the same purpose. The Pap smear involves placing the sample cells directly onto a microscopic slide. With liquid-based cytology, the sample is placed into a vial,2,7 where a portion is processed in preservative liquid under light microscopy for review by a cytologist.7 The remainder of the cell sample can be tested for HPV and STIs.
DNA testing for high-risk HPV subtypes is available and has been incorporated into the cervical cancer screening guidelines. The guidelines recommend HPV DNA testing as a co-test to be performed with cytology in women older than 30.2,24 The recommendation for HPV DNA testing in this age-group reflects greater concern that older women’s immune systems will not clear the virus, leaving these women at higher risk for developing CIN and cancer.25,26 HPV-16 and HPV-18 genotyping is available and is recommended in women 30 or older whose co-testing reveals normal cytology in the presence of a high-risk HPV subtype.27
Additionally, HPV DNA testing is used to triage cytology that reveals atypical squamous cells of undetermined significance. HPV DNA testing is not performed routinely in younger women because of the high likelihood that their immune systems will clear the virus. Genetic testing for the 3q26 gene amplification is available but has not yet been incorporated into the screening guidelines.27
Screening Guidelines
In September 2012, the American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology developed a new set of cervical cancer screening guidelines.28 The US Preventive Services Task Force (USPSTF) and American College of Obstetricians and Gynecologists (ACOG) have also created standard recommendations for cervical cancer screening (see Table 32,28-34). Although ACOG’s standards have varied from those of other organizations in the past, their 2012 guidelines now align closely.
All these organizations recommend that cervical screening begin at age 21 for all women, regardless of the age of sexual initiation.31 Many young women who acquire an HPV infection will clear the infection within the first one to two years. Eliminating screening of women younger than 21 will help to avoid unnecessary biopsies and invasive treatment.29-32
All the guidelines include general recommendations for screening; however, each organization has made exceptions for patients at higher risk for developing cervical cancer.2,28-34 ASCCP has also developed algorithms for management of the multitude of abnormal cervical cancer screening results. These algorithms are available through the ASCCP Web site (www.asccp.org).
Vaccinations
HPV vaccines developed in recent years are now helping to prevent HPV infections from occurring. Currently, the FDA has approved the use of two HPV vaccines: a quadrivalent recombinant HPV vaccine (Gardasil) in 2006 and a bivalent HPV vaccine in 2009 (Cervarix).2 The quadrivalent vaccine was approved for girls and women ages 9 to 26 and protects against HPV subtypes 6 and 11—two low-risk HPV subtypes that can cause genital warts—and subtypes 16 and 18—two high-risk HPV subtypes that can cause cervical cancer. This vaccine is given in a three-dose series at months 0, 2, and 6, and should be initiated before women become sexually active for maximum effectiveness.22,35 The quadrivalent vaccine received additional FDA approval for administration in boys and men ages 9 to 26 in 2010.36
The bivalent vaccine is given in a three-dose series as well and is approved for girls and women ages 9 to 25.37 This vaccine offers protection against high-risk HPV subtypes 16 and 18.38
Recent trials have shown that both the quadrivalent and bivalent vaccines are more than 90% effective in preventing the development of precancerous cells from HPV subtypes 16 and 18.2 Although these vaccines provide protection against the two most oncogenic HPV subtypes, routine cytologic testing is still recommended in patients who receive the vaccines because they do not protect against all high-risk subtypes.2,35,38
On the next page: Diagnosis and staging >>
DIAGNOSIS AND STAGING
The Bethesda staging system provides uniform terminology to classify all abnormal Pap smears and cytology reports (see Table 434). Patients with abnormal cytology typically undergo a colposcopic examination to further evaluate the abnormality. During a colposcopy, the clinician examines the cervix with the use of a lighted microscope, known as a colposcope, which magnifies the cells of the cervix and allows for localized sampling of abnormal-appearing areas through punch biopsy.7,9 The area of focus is the transformation zone because of its known vulnerability to HPV infections. A colposcopy is considered inadequate if the SCJ, and therefore the transformation zone, is not fully visualized.
Acetic acid is applied to the cervix during colposcopy, and any dysplastic cells present take up the acid and turn white, a process known as acetowhitening. Indications for a punch biopsy include acetowhitening, leukoplakia, and abnormal vasculature marked by punctation, bizarre-appearing vessels, or the appearance of a mosaic pattern.16 Indications for sampling of the endocervical canal through endocervical curettage include inadequate colposcopy, presence of a lesion that extends beyond the view of the colposcope, or atypical glandular cells on cytology.16 The biopsy results help to determine if a diagnostic conization is needed for further evaluation of the lesion.16
After a cervical biopsy is performed, the abnormal cells may be classified as CIN 1, 2, or 3 or carcinoma in situ (see Table 516). These stages of intraepithelial neoplasia are determined by the depth to which abnormal, immature cells have invaded the cervical epithelium.16 CIN 2 and 3 are more likely to develop into cervical cancer if they are not properly treated.5,16,17 The majority of CIN 1 lesions do not progress into cancer and typically resolve on their own or are cleared by the patient’s immune system. While most CIN 1 lesions are caused by high-risk HPV, these may be less oncogenic subtypes.7,17
The two most common histologic subtypes of cervical cancer are squamous cell carcinoma and adenocarcinoma. Squamous cell carcinoma comprises more than 70% of cervical cancers, while adenocarcinoma makes up approximately 25%.39 Neuroendocrine, small cell, and mixed-cell carcinomas make up the remainder of cervical cancers.9 Squamous cell carcinoma arises from the squamocolumnar junction or ectocervix, and adenocarcinoma tends to arise from the glandular cells of the endocervix.7,9,17 Studies show that adenocarcinoma is slowly becoming more prevalent in the US than squamous cell carcinoma.9
Once a diagnosis of cervical cancer has been established, additional testing and procedures are performed to rule out lymph node and organ involvement.9,16 The International Federation of Gynecology and Oncology system is then used to clinically stage the cervical cancer40 (see Table 67,40,41). This staging system, updated in 2009, is based solely on clinical examination findings. Once cervical cancer is staged, measures are taken to assess which treatment option is most appropriate.
On the next page: Treatment modalities >>
TREATMENT MODALITIES
Treatment strategies for CIN correspond to the degree of neoplasia and adequacy of colposcopy, and tend to vary among clinicians. For invasive cervical cancer, findings such as lymph node dissemination and adjacent organ involvement are key factors in determining which therapy will be selected.16
Cervical Intraepithelial Neoplasia
The treatment options for CIN can be divided into two categories, ablative and excisional. These techniques may be used only when invasive cervical cancer has been excluded.16,42 Cryotherapy and laser ablation are two common ablative techniques. One disadvantage of ablative techniques is that abnormal tissue is destroyed during the procedure, making it impossible for a tissue specimen to be collected and sent for additional evaluation.42,43 Cold knife conization, laser cone excision, and loop electrosurgical excision procedure (LEEP) are all excisional treatments that allow tissue to be collected and further evaluated.44 Although all these procedures can be used for any stage of CIN, excisional procedures are preferred for CIN 2 and CIN 3 lesions because moderate and severe intraepithelial neoplasias have a higher incidence of undetected microinvasive disease within the endocervical canal. Ablative therapy is generally reserved for treating low-grade CIN or small, focal high-grade lesions.42
Ablative techniques. Cryotherapy was the first outpatient treatment developed for CIN and is still widely used due to its ease, low cost, and low complication rate.44 It is an in-office procedure that utilizes nitrous oxide and carbon dioxide to freeze and ablate abnormal cells on the ectocervix. The procedure involves a freeze-thaw-freeze technique that has improved the efficacy of this treatment.16 The main side effects of cryotherapy are mild cramping and copious watery discharge that can last for up to 4 weeks postprocedure.42,45
Laser ablation is not used as commonly as cryosurgery, but is a viable option for women with large CIN lesions or women unwilling to undergo a LEEP. This procedure is performed under either local or general anesthesia and involves the use of a carbon dioxide laser under colposcopic guidance to ablate the transformation zone of the cervix.45 Laser ablation completely destroys the lesion while causing minimal damage to the surrounding, unaffected tissue. After treatment, patients may experience vaginal discharge for approximately 1 to 2 weeks.44,45 Overall, this technique is very expensive and requires a substantial amount of training to achieve maximum effectiveness.44
Excisional techniques. The LEEP is the procedure of choice for treating moderate and high-grade lesions. LEEP uses a wire loop electrode to excise the transformation zone of the cervix. Acetic acid or an iodine solution known as Lugol’s solution is applied to the cervix to delineate the margins of the lesion.42 The majority of these procedures can be done under local anesthesia, but on occasion sedation may be required.44 Complications, although rare, include post-treatment bleeding, infection, cervical stenosis, and cervical incompetence.45
Excisional conization is a procedure that can be performed with a scalpel (cold knife conization) or with a laser (laser cone excision).45 This procedure involves the removal of a cone-shaped portion of the cervix. Following the conization, endocervical curettage may be performed to acquire tissue samples and assess the endocervical canal. Also known as a cone biopsy, the procedure is typically performed under sedation. The types of complications associated with excisional conization are the same as those for LEEP, but the complication rate is higher when cone biopsy is performed.16,45
CERVICAL CANCER
Treatment methods for cervical cancer can be classified according to one of three disease stages: early-stage (IA2-IIA2), locally advanced (IIB-IVA), and advanced disease (IVB). Common treatment options for cervical cancer include radical hysterectomy with pelvic lymphadenectomy, radiation, and chemotherapy.9,17 Patients diagnosed with early-stage disease typically undergo a radical hysterectomy with pelvic lymphadenectomy. Cervical conization is an alternative option for young women who want to preserve fertility and wish to avoid a hysterectomy.45 Women who undergo surgery and are found to have more extensive disease or parametrial invasion are offered adjuvant radiation therapy.9
Patients diagnosed with locally advanced cancer are typically treated with radiation, chemotherapy, or both.17,45 The combination of radiation and chemotherapy in cervical cancer has been shown to increase survival rates in late-stage disease.7 Those diagnosed with advanced or disseminated disease are treated with palliative radiation. Overall, the five-year survival rates for patients diagnosed with early-stage cervical cancer who receive appropriate treatment have proven to be excellent7 (see Table 77,40,46).
On the next page: Conclusion >>
CONCLUSION
The incidence and prevalence of cervical cancer continue to decline in the US; the majority of cases that arise are due to the lack of knowledge and availability of screening services in underserved areas. Increasing awareness and developing clinics for these under-screened populations would most likely contribute to a further decline in the incidence of CIN and cervical cancer.
Once diagnosed with an HPV infection, women should be informed that the body generally clears these infections within 1 to 2 years. Persistence of a high-risk HPV infection greatly increases the risk for neoplastic tissue transformation. These women need to be followed diligently so that treatment can be implemented at the first signs of a CIN 2 or higher grade lesion. Ultimately, implementing adequate screening techniques allows for early detection of CIN and prevention of cervical cancer.
CE/CME No: CR-1309
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the central role that human papillomavirus (HPV) infection plays in the development of cervical intraepithelial neoplasia (CIN) and cervical cancer.
• Instruct patients on contributing cofactors of HPV infection and cervical cancer, including tobacco use, parity, use of oral contraceptives, co-infection with HIV, and immunosuppression.
• Describe the current recommendations for cervical cancer screening from professional societies, national health organizations, and federal agencies, including age-appropriate screening for cytology and high-risk HPV.
• Explain the timing and administration schedules of the currently available HPV vaccines and the patient populations for which the vaccines have been approved.
• Discuss the ablative and excisional procedures used to treat CIN and the treatment options for cervical cancer.
FACULTY
Heather P. Adams is an Assistant Professor/Clinical Coordinator in the Physician Assistant Program at Gannon University in Erie, Pennsylvania; in the program, Erica L. Carnright is a Physician Assistant student on clinical rotations.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Improved understanding of the central role of human papillomavirus (HPV) in cervical carcinogenesis has led to the development of vaccines and DNA testing for high-risk HPV subtypes. But age-appropriate cytologic screening remains the cornerstone in the prevention and early detection of cervical intraepithelial neoplasia (CIN) and cervical cancer. With prompt diagnosis and treatment of both CIN and early-stage cervical cancer, the prognosis for this disease is excellent.
Cervical cancer is the third most common cancer in women worldwide.1 More than 12,000 women are diagnosed with cervical cancer annually in the United States, with nearly 4,000 cervical cancer deaths reported each year.2-4 Globally, cervical cancer accounts for more than 529,000 new cancer cases and more than 275,000 cancer deaths in women annually, with more than 80% of these cases occurring in developing countries.1,5-7 Over the past 50 years, overall cervical cancer incidence and mortality have declined by more than 70% in the US, largely due to the development of the Papanicolaou screening test (Pap smear).7
Cervical cancer develops from precancerous, or neoplastic, cells of the cervix. Known as cervical intraepithelial neoplasia (CIN), these precancerous lesions are caused by infection with the human papillomavirus (HPV), the main etiologic factor in the development of cervical cancer. The progression from CIN to invasive cervical cancer is typically slow. Because of the long natural history of cervical cancer, screening and preventive measures, when appropriately implemented, can identify precancerous cells before they progress to cervical cancer.
EPIDEMIOLOGY AND RISK FACTORS
In 2013, approximately 12,340 new cases of cervical cancer will be diagnosed in the US, and 4,030 US women will die as a result of cervical cancer, according to National Cancer Institute estimates.8 Although the incidence rate of cervical cancer in the US has declined significantly, medically underserved populations remain disproportionately affected, with more than 60% of new cases occurring in underserved areas or underscreened populations.6 Epidemiologic studies have also shown higher incidence and mortality among minority populations.9 The incidence of cervical cancer is 30% higher among African American than white women, and the mortality rate is twice as high.9 The incidence of cervical cancer is highest among Hispanics.8
Although many risk factors contribute to the development of cervical cancer, HPV infection is the most important (see Table 12,7,9). HPV has been detected in up to 99% of all invasive cervical cancers, and its presence is necessary for oncogenesis to occur.10 HPV is the most common type of sexually transmitted infection (STI) in the US, with 75% to 80% of sexually active adults acquiring an HPV infection before age 50.11 It is estimated that a woman has a 10% lifetime risk for being infected with HPV, and this risk increases by 15% to 25% with each new sexual partner.7
Studies have shown that cigarette smoking contributes to the development of cervical cancer, with both previous and current smokers having a two- to threefold increased risk for high-grade CIN and cervical cancer, compared with never-smokers.5,9,12 Although the exact cause of this association has not been determined, carcinogens from cigarettes have been found within the cervical mucosa, which may cause alterations in the tissue.2,7
Long-term (≥ 5 years) use of oral contraceptive pills (OCP) by patients with an HPV cervical infection has been established as a risk factor for cervical cancer. It has been shown that the use of OCP causes an increase in HPV gene expression.5 In a study that compared two groups of women who tested positive for HPV DNA, the group that used OCP had a fourfold greater risk for cervical cancer than the group that did not use OCP.2,9,13
Parity, particularly multiparity, and HIV co-infection have also been implicated as risk factors for cervical cancer. The relationship between parity and cervical cancer likely results from the presence of the transformation zone on the ectocervix and increased squamous metaplasia during pregnancy, both of which make cervical tissue more vulnerable to HPV infection.14 HIV co-infection increases the risk for cervical cancer secondary to its immunosuppressive effects; women with both HIV and high-risk HPV infections are four times more likely to develop cervical cancer.15
On the next page: Cervical anatomy >>
CERVICAL ANATOMY
The cervix is the narrow, fibromuscular neck that makes up the lower portion of the uterus. The endocervix is the cervical canal, and the ectocervix extends inferiorly into the vagina.7 The internal os is the opening of the cervix into the uterus, and the external os is the opening of the cervix into the vagina. The endocervix is lined with glandular columnar epithelium. This tissue can extend beyond the external os onto the ectocervix, and when this occurs it is known as cervical ectopy.16 The surface of the cervix lying between the glandular tissue and the vaginal wall is comprised of stratified nonkeratinizing squamous epithelium.7 The squamocolumnar junction (SCJ) is defined as the location where the squamous and glandular cells meet.17 Adjacent to the SCJ is the transformation zone, an area vulnerable to HPV infection where glandular cells are actively undergoing squamous metaplasia.17
The location of the SCJ varies depending on age and hormonal changes. In prepubertal females, the SCJ is close to the external os, but in women of reproductive age, the SCJ moves away from the external os onto the surface of the ectocervix. This change in the location of the SCJ occurs due to increased estrogen levels following menarche, which cause the endocervical canal to elongate.7,17 A satisfactory Pap smear for cervical cytology includes cells from the transformation zone, as well as the ectocervix and endocervix.
HUMAN PAPILLOMAVIRUS
The human papillomavirus is a nonenveloped, double-stranded DNA virus that is known to cause abnormalities in skin cells. This virus is predominantly spread through sexual contact via skin-to-skin transmission. There are more than 100 known subtypes in the HPV family, of which 40 subtypes have been recognized to cause genital tract disease. Of these 40 subtypes, 15 have been identified to be oncogenic.9,18,19
HPV subtypes are separated into low-risk and high-risk categories (see Table 25,7,18). The low-risk subtypes cause either no cellular change, low-grade intraepithelial neoplasia, or condyloma. The high-risk subtypes are associated with the development of CIN and cancer. Of the 15 high-risk subtypes, HPV-16 and HPV-18 are the most oncogenic, followed by HPV-31 and HPV-45. HPV-16 is implicated in up to 70% of cervical cancers, HPV-18 in up to 20%, and HPV-31 and HPV-45 in up to 10%.5,9,20 Of the low-risk HPV subtypes, HPV-6 and HPV-11 cause 90% of condyloma infections.16,21
After HPV infection is contracted, the virus migrates into the squamous epithelial mucosa of the cervix, where it is capable of altering cells and causing the development of precancerous properties. This typically happens within the transformation zone, which is vulnerable to HPV infection.17 Depending on the state of the host’s immune system, many of these HPV infections clear without intervention. It is estimated that approximately 70% of HPV infections resolve spontaneously within one year, and 90% resolve within two years.5 HPV infections that persist beyond two years are more likely to lead to the development of CIN and, ultimately, cervical cancer, if left untreated.22 There is an association between genetic amplification (extra copies) of gene 3q26 and progression of CIN to cervical cancer, but ultimately a high-risk HPV subtype must be involved for cervical cancer to develop.23
On the next page: Clinical manifestations, screening, and prevention >>
CLINICAL MANIFESTATIONS
Due to the widespread use of cervical cytology, many cases of CIN and cervical cancer are diagnosed well before symptoms develop. In the early stages of cervical cancer, most women are asymptomatic, although some women may present with a watery, blood-tinged, or malodorous vaginal discharge.7 Any type of abnormal bleeding, whether it is between menstrual cycles, postcoital, or postmenopause, warrants an evaluation for pathologic processes, including but not limited to cervical cancer. Patients with late-stage cervical cancer may present with complaints of pelvic pain and painful intercourse due to the enlargement of a cervical mass. Vaginal discharge may change in consistency from watery to purulent and become foul-smelling due to cervical tissue necrosis.7,9,22 In some cases of advanced invasive cervical cancer, patients will experience hematuria or rectal bleeding secondary to tumor invasion through the bladder or rectal wall.9 Other nonspecific signs and symptoms of cervical cancer include unexplained weight loss accompanied by nausea or vomiting, as well as loss of appetite.7,9
SCREENING AND PREVENTION
Widespread use of the Pap test since the 1950s has led to marked reductions in the incidence of cervical cancer. The Pap test enables clinicians to screen for and detect CIN. More recently, liquid-based cytology has been utilized for the same purpose. The Pap smear involves placing the sample cells directly onto a microscopic slide. With liquid-based cytology, the sample is placed into a vial,2,7 where a portion is processed in preservative liquid under light microscopy for review by a cytologist.7 The remainder of the cell sample can be tested for HPV and STIs.
DNA testing for high-risk HPV subtypes is available and has been incorporated into the cervical cancer screening guidelines. The guidelines recommend HPV DNA testing as a co-test to be performed with cytology in women older than 30.2,24 The recommendation for HPV DNA testing in this age-group reflects greater concern that older women’s immune systems will not clear the virus, leaving these women at higher risk for developing CIN and cancer.25,26 HPV-16 and HPV-18 genotyping is available and is recommended in women 30 or older whose co-testing reveals normal cytology in the presence of a high-risk HPV subtype.27
Additionally, HPV DNA testing is used to triage cytology that reveals atypical squamous cells of undetermined significance. HPV DNA testing is not performed routinely in younger women because of the high likelihood that their immune systems will clear the virus. Genetic testing for the 3q26 gene amplification is available but has not yet been incorporated into the screening guidelines.27
Screening Guidelines
In September 2012, the American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology developed a new set of cervical cancer screening guidelines.28 The US Preventive Services Task Force (USPSTF) and American College of Obstetricians and Gynecologists (ACOG) have also created standard recommendations for cervical cancer screening (see Table 32,28-34). Although ACOG’s standards have varied from those of other organizations in the past, their 2012 guidelines now align closely.
All these organizations recommend that cervical screening begin at age 21 for all women, regardless of the age of sexual initiation.31 Many young women who acquire an HPV infection will clear the infection within the first one to two years. Eliminating screening of women younger than 21 will help to avoid unnecessary biopsies and invasive treatment.29-32
All the guidelines include general recommendations for screening; however, each organization has made exceptions for patients at higher risk for developing cervical cancer.2,28-34 ASCCP has also developed algorithms for management of the multitude of abnormal cervical cancer screening results. These algorithms are available through the ASCCP Web site (www.asccp.org).
Vaccinations
HPV vaccines developed in recent years are now helping to prevent HPV infections from occurring. Currently, the FDA has approved the use of two HPV vaccines: a quadrivalent recombinant HPV vaccine (Gardasil) in 2006 and a bivalent HPV vaccine in 2009 (Cervarix).2 The quadrivalent vaccine was approved for girls and women ages 9 to 26 and protects against HPV subtypes 6 and 11—two low-risk HPV subtypes that can cause genital warts—and subtypes 16 and 18—two high-risk HPV subtypes that can cause cervical cancer. This vaccine is given in a three-dose series at months 0, 2, and 6, and should be initiated before women become sexually active for maximum effectiveness.22,35 The quadrivalent vaccine received additional FDA approval for administration in boys and men ages 9 to 26 in 2010.36
The bivalent vaccine is given in a three-dose series as well and is approved for girls and women ages 9 to 25.37 This vaccine offers protection against high-risk HPV subtypes 16 and 18.38
Recent trials have shown that both the quadrivalent and bivalent vaccines are more than 90% effective in preventing the development of precancerous cells from HPV subtypes 16 and 18.2 Although these vaccines provide protection against the two most oncogenic HPV subtypes, routine cytologic testing is still recommended in patients who receive the vaccines because they do not protect against all high-risk subtypes.2,35,38
On the next page: Diagnosis and staging >>
DIAGNOSIS AND STAGING
The Bethesda staging system provides uniform terminology to classify all abnormal Pap smears and cytology reports (see Table 434). Patients with abnormal cytology typically undergo a colposcopic examination to further evaluate the abnormality. During a colposcopy, the clinician examines the cervix with the use of a lighted microscope, known as a colposcope, which magnifies the cells of the cervix and allows for localized sampling of abnormal-appearing areas through punch biopsy.7,9 The area of focus is the transformation zone because of its known vulnerability to HPV infections. A colposcopy is considered inadequate if the SCJ, and therefore the transformation zone, is not fully visualized.
Acetic acid is applied to the cervix during colposcopy, and any dysplastic cells present take up the acid and turn white, a process known as acetowhitening. Indications for a punch biopsy include acetowhitening, leukoplakia, and abnormal vasculature marked by punctation, bizarre-appearing vessels, or the appearance of a mosaic pattern.16 Indications for sampling of the endocervical canal through endocervical curettage include inadequate colposcopy, presence of a lesion that extends beyond the view of the colposcope, or atypical glandular cells on cytology.16 The biopsy results help to determine if a diagnostic conization is needed for further evaluation of the lesion.16
After a cervical biopsy is performed, the abnormal cells may be classified as CIN 1, 2, or 3 or carcinoma in situ (see Table 516). These stages of intraepithelial neoplasia are determined by the depth to which abnormal, immature cells have invaded the cervical epithelium.16 CIN 2 and 3 are more likely to develop into cervical cancer if they are not properly treated.5,16,17 The majority of CIN 1 lesions do not progress into cancer and typically resolve on their own or are cleared by the patient’s immune system. While most CIN 1 lesions are caused by high-risk HPV, these may be less oncogenic subtypes.7,17
The two most common histologic subtypes of cervical cancer are squamous cell carcinoma and adenocarcinoma. Squamous cell carcinoma comprises more than 70% of cervical cancers, while adenocarcinoma makes up approximately 25%.39 Neuroendocrine, small cell, and mixed-cell carcinomas make up the remainder of cervical cancers.9 Squamous cell carcinoma arises from the squamocolumnar junction or ectocervix, and adenocarcinoma tends to arise from the glandular cells of the endocervix.7,9,17 Studies show that adenocarcinoma is slowly becoming more prevalent in the US than squamous cell carcinoma.9
Once a diagnosis of cervical cancer has been established, additional testing and procedures are performed to rule out lymph node and organ involvement.9,16 The International Federation of Gynecology and Oncology system is then used to clinically stage the cervical cancer40 (see Table 67,40,41). This staging system, updated in 2009, is based solely on clinical examination findings. Once cervical cancer is staged, measures are taken to assess which treatment option is most appropriate.
On the next page: Treatment modalities >>
TREATMENT MODALITIES
Treatment strategies for CIN correspond to the degree of neoplasia and adequacy of colposcopy, and tend to vary among clinicians. For invasive cervical cancer, findings such as lymph node dissemination and adjacent organ involvement are key factors in determining which therapy will be selected.16
Cervical Intraepithelial Neoplasia
The treatment options for CIN can be divided into two categories, ablative and excisional. These techniques may be used only when invasive cervical cancer has been excluded.16,42 Cryotherapy and laser ablation are two common ablative techniques. One disadvantage of ablative techniques is that abnormal tissue is destroyed during the procedure, making it impossible for a tissue specimen to be collected and sent for additional evaluation.42,43 Cold knife conization, laser cone excision, and loop electrosurgical excision procedure (LEEP) are all excisional treatments that allow tissue to be collected and further evaluated.44 Although all these procedures can be used for any stage of CIN, excisional procedures are preferred for CIN 2 and CIN 3 lesions because moderate and severe intraepithelial neoplasias have a higher incidence of undetected microinvasive disease within the endocervical canal. Ablative therapy is generally reserved for treating low-grade CIN or small, focal high-grade lesions.42
Ablative techniques. Cryotherapy was the first outpatient treatment developed for CIN and is still widely used due to its ease, low cost, and low complication rate.44 It is an in-office procedure that utilizes nitrous oxide and carbon dioxide to freeze and ablate abnormal cells on the ectocervix. The procedure involves a freeze-thaw-freeze technique that has improved the efficacy of this treatment.16 The main side effects of cryotherapy are mild cramping and copious watery discharge that can last for up to 4 weeks postprocedure.42,45
Laser ablation is not used as commonly as cryosurgery, but is a viable option for women with large CIN lesions or women unwilling to undergo a LEEP. This procedure is performed under either local or general anesthesia and involves the use of a carbon dioxide laser under colposcopic guidance to ablate the transformation zone of the cervix.45 Laser ablation completely destroys the lesion while causing minimal damage to the surrounding, unaffected tissue. After treatment, patients may experience vaginal discharge for approximately 1 to 2 weeks.44,45 Overall, this technique is very expensive and requires a substantial amount of training to achieve maximum effectiveness.44
Excisional techniques. The LEEP is the procedure of choice for treating moderate and high-grade lesions. LEEP uses a wire loop electrode to excise the transformation zone of the cervix. Acetic acid or an iodine solution known as Lugol’s solution is applied to the cervix to delineate the margins of the lesion.42 The majority of these procedures can be done under local anesthesia, but on occasion sedation may be required.44 Complications, although rare, include post-treatment bleeding, infection, cervical stenosis, and cervical incompetence.45
Excisional conization is a procedure that can be performed with a scalpel (cold knife conization) or with a laser (laser cone excision).45 This procedure involves the removal of a cone-shaped portion of the cervix. Following the conization, endocervical curettage may be performed to acquire tissue samples and assess the endocervical canal. Also known as a cone biopsy, the procedure is typically performed under sedation. The types of complications associated with excisional conization are the same as those for LEEP, but the complication rate is higher when cone biopsy is performed.16,45
CERVICAL CANCER
Treatment methods for cervical cancer can be classified according to one of three disease stages: early-stage (IA2-IIA2), locally advanced (IIB-IVA), and advanced disease (IVB). Common treatment options for cervical cancer include radical hysterectomy with pelvic lymphadenectomy, radiation, and chemotherapy.9,17 Patients diagnosed with early-stage disease typically undergo a radical hysterectomy with pelvic lymphadenectomy. Cervical conization is an alternative option for young women who want to preserve fertility and wish to avoid a hysterectomy.45 Women who undergo surgery and are found to have more extensive disease or parametrial invasion are offered adjuvant radiation therapy.9
Patients diagnosed with locally advanced cancer are typically treated with radiation, chemotherapy, or both.17,45 The combination of radiation and chemotherapy in cervical cancer has been shown to increase survival rates in late-stage disease.7 Those diagnosed with advanced or disseminated disease are treated with palliative radiation. Overall, the five-year survival rates for patients diagnosed with early-stage cervical cancer who receive appropriate treatment have proven to be excellent7 (see Table 77,40,46).
On the next page: Conclusion >>
CONCLUSION
The incidence and prevalence of cervical cancer continue to decline in the US; the majority of cases that arise are due to the lack of knowledge and availability of screening services in underserved areas. Increasing awareness and developing clinics for these under-screened populations would most likely contribute to a further decline in the incidence of CIN and cervical cancer.
Once diagnosed with an HPV infection, women should be informed that the body generally clears these infections within 1 to 2 years. Persistence of a high-risk HPV infection greatly increases the risk for neoplastic tissue transformation. These women need to be followed diligently so that treatment can be implemented at the first signs of a CIN 2 or higher grade lesion. Ultimately, implementing adequate screening techniques allows for early detection of CIN and prevention of cervical cancer.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69-90.
2. Whyte J. HPV and cervical cancer: latest developments. Consultant. 2012;52:555-560.
3. CDC. Cervical cancer statistics. www.cdc.gov/cancer/cervical/statistics/. Accessed July 23, 2013.
4. National Cancer Institute. Human papillomavirus (HPV) vaccines. www.cancer.gov/cancertopics/factsheet/prevention/HPV-vaccine. Accessed July 23, 2013.
5. de Freitas AC, Gurgel AP, Chagas BS, et al. Susceptibility to cervical cancer: an overview. Gynecol Oncol. 2012;126:304-311.
6. Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531-2542.
7. Leaver D, Labonte G. HPV and cervical cancer. Radiation Therapist. 2010;19:27-45.
8. National Cancer Institute. SEER stat facts sheets: Cervix uteri cancer. http://seer.cancer.gov/statfacts/html/cervix.html#incidence-mortality. Accessed July 5, 2013.
9. Lea JS, Lin KY. Cervical cancer. Obstet Gynecol Clin North Am. 2012;39:233-253.
10. McKeever AE. Cervical cancer risk among college-age women: a review of the literature. SGNO J. 2010;20:6-12.
11. Frumovitz M. Invasive cervical cancer: epidemiology, risk factors, clinical manifestations, and diagnosis. UpToDate. Goff B, Falk SJ, eds. UpToDate, Waltham. MA, 2013. www.uptodate.com/contents/invasive-cervical-cancer-epidemiology-risk-factors-clinical-manifestations-and-diagnosis?detectedLanguage=en&source=search_result&search=Lifetime+prevalence+of+HPV+infection&selectedTitle=10%7E150&provider=noProvider. Accessed July 23, 2013.
12. Collins S, Rollason TP, Young LS, Woodman CB. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: a longitudinal study. Eur J Cancer. 2010;46:405-411.
13. Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085-1092.
14. Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359(9312):1093-1101.
15. Kalra S. Studies examine relationship between HIV and cervical cancer in women. The AIDS Beacon. 2010. www.aidsbeacon.com/news/2010/07/23/studies-examine-relationship-between-hiv-and-cervical-cancer-in-women-aids-2010/. Accessed July 6, 2013.
16. Holschneider CH. Premalignant and malignant disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics and Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:807-808, 811-816, 820.
17. Rajaram S, Chitrathara K, Maheshwari A. Cervical Cancer: Contemporary Management. 1st ed. New Dehli, India: Jaypee Brothers Medical Publishers; 2012.
18. Carter JR, Ding Z, Rose BR. HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol. 2011;51:103-108.
19. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5-10.
20. Khachikyan I, Stratton P. Benign disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics & Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:650.
21. CDC. 2010 sexually transmitted disease surveillance. www.cdc.gov/std/stats10/other.htm. Accessed July 23, 2013.
22. Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82:1209-1214.
23. Verri A, Jalali GR, Cecchini G, et al. Significant progression of uterine cervical epithelial lesion accompanied by marked increase in 3q26 gene amplification. Lab Med. 2010;42:134-136.
24. Schwaiger C, Aruda M, LaCoursiere S, Rubin R. Current guidelines for cervical cancer screening. J Am Acad Nurse Prac. 2012;24:417-424.
25. CDC. Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older. www.cdc.gov/cancer/hpv/basic_info/screening/cervical_screening.htm. Accessed July 5, 2013.
26. Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103: 368-383.
27. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updates consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1-S27.
28. American Society for Colposcopy and Cervical Pathology. Cervical cancer screening recommendations, 2012. www.asccp.org/Portals/9/docs/pdfs/Practice%20Management/ASCCP_Cervical_Cancer_Screening_Recommendations.pdf#zoom=80. Accessed July 6, 2013.
29. American Cancer Society. New screening guidelines for cervical cancer. March 14, 2012. www.cancer.org/cancer/news/news/new-screening-guidelines-for-cervical-cancer. Accessed July 23, 2013.
30. American Congress of Obstetricians and Gynecologists. USPSTF updated cervical cancer screening. www.acog.org/~/media/Districts/District%20II/PDFs/USPSTF_Cervical_Ca_Screening_Guidelines.pdf?dmc=1&ts=20130723T1510069242. Accessed July 23, 2013.
31. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed July 5, 2013.
32. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
33. US Preventive Services Task Force. Screening for cervical cancer. Current recommendation. www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm#clinical. Accessed July 6, 2013.
34. American College of Obstetricians and Gynecologists; Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120:1222-1238.
35. Warman J. Cervical cancer screening in young women: saving lives with prevention and detection. Oncol Nurs Forum. 2010;37:33-38.
36. Gardasil [package insert]. Whitehouse Station, NJ: Merck & Co; 2011.
37. Cervarix [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
38. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
39. Morrison RS, Moody R, Shelton M. Pap smear rates: predictor of cervical cancer mortality disparity? Online J Rural Nurs Health Care. 2010;10:21-27.
40. National Cancer Institute. Cervical cancer treatment. www.cancer.gov/cancertopics/pdq/treatment/cervical/HealthProfessional/page1/AllPages#2.
41. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-104.
42. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99. management of abnormal cervical cytology and histology. J Obstet Gynecol. 2008;112:1419-1444.
43. Bowring J, Tulloch I, Phadnis SV, et al. Secondary excision for cervical intraepithelial neoplasia: an evaluation of two treatment methods. J Obstet Gynaecol. 2010;30:511-514.
44. Jhingran A. Neoplasms of the cervix. In: Hong KW, Bast RC Jr, Hait WN, et al, eds. Holland-Frei Cancer Medicine. 8th ed. Ontario, Canada: BC Decker; 2010:1304-1312.
45. Kim SH. Preinvasive disease of the lower genital tract. In: Chu CS, Rubin SC, eds. Manual of Gynecologic Oncology. 1st ed. Hackensack, NJ: World Scientific Publishing Company; 2011:80-87.
46. American Cancer Society. Cervical cancer key statistics. http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statistics. Accessed July 5, 2013.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69-90.
2. Whyte J. HPV and cervical cancer: latest developments. Consultant. 2012;52:555-560.
3. CDC. Cervical cancer statistics. www.cdc.gov/cancer/cervical/statistics/. Accessed July 23, 2013.
4. National Cancer Institute. Human papillomavirus (HPV) vaccines. www.cancer.gov/cancertopics/factsheet/prevention/HPV-vaccine. Accessed July 23, 2013.
5. de Freitas AC, Gurgel AP, Chagas BS, et al. Susceptibility to cervical cancer: an overview. Gynecol Oncol. 2012;126:304-311.
6. Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531-2542.
7. Leaver D, Labonte G. HPV and cervical cancer. Radiation Therapist. 2010;19:27-45.
8. National Cancer Institute. SEER stat facts sheets: Cervix uteri cancer. http://seer.cancer.gov/statfacts/html/cervix.html#incidence-mortality. Accessed July 5, 2013.
9. Lea JS, Lin KY. Cervical cancer. Obstet Gynecol Clin North Am. 2012;39:233-253.
10. McKeever AE. Cervical cancer risk among college-age women: a review of the literature. SGNO J. 2010;20:6-12.
11. Frumovitz M. Invasive cervical cancer: epidemiology, risk factors, clinical manifestations, and diagnosis. UpToDate. Goff B, Falk SJ, eds. UpToDate, Waltham. MA, 2013. www.uptodate.com/contents/invasive-cervical-cancer-epidemiology-risk-factors-clinical-manifestations-and-diagnosis?detectedLanguage=en&source=search_result&search=Lifetime+prevalence+of+HPV+infection&selectedTitle=10%7E150&provider=noProvider. Accessed July 23, 2013.
12. Collins S, Rollason TP, Young LS, Woodman CB. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: a longitudinal study. Eur J Cancer. 2010;46:405-411.
13. Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085-1092.
14. Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359(9312):1093-1101.
15. Kalra S. Studies examine relationship between HIV and cervical cancer in women. The AIDS Beacon. 2010. www.aidsbeacon.com/news/2010/07/23/studies-examine-relationship-between-hiv-and-cervical-cancer-in-women-aids-2010/. Accessed July 6, 2013.
16. Holschneider CH. Premalignant and malignant disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics and Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:807-808, 811-816, 820.
17. Rajaram S, Chitrathara K, Maheshwari A. Cervical Cancer: Contemporary Management. 1st ed. New Dehli, India: Jaypee Brothers Medical Publishers; 2012.
18. Carter JR, Ding Z, Rose BR. HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol. 2011;51:103-108.
19. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5-10.
20. Khachikyan I, Stratton P. Benign disorders of the uterine cervix. In: Decherney AH, Nathan L, Laufer N, Roman AS, eds. Current Diagnosis and Treatment: Obstetrics & Gynecology. 11th ed. New York, NY: McGraw-Hill; 2013:650.
21. CDC. 2010 sexually transmitted disease surveillance. www.cdc.gov/std/stats10/other.htm. Accessed July 23, 2013.
22. Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82:1209-1214.
23. Verri A, Jalali GR, Cecchini G, et al. Significant progression of uterine cervical epithelial lesion accompanied by marked increase in 3q26 gene amplification. Lab Med. 2010;42:134-136.
24. Schwaiger C, Aruda M, LaCoursiere S, Rubin R. Current guidelines for cervical cancer screening. J Am Acad Nurse Prac. 2012;24:417-424.
25. CDC. Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older. www.cdc.gov/cancer/hpv/basic_info/screening/cervical_screening.htm. Accessed July 5, 2013.
26. Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103: 368-383.
27. Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updates consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1-S27.
28. American Society for Colposcopy and Cervical Pathology. Cervical cancer screening recommendations, 2012. www.asccp.org/Portals/9/docs/pdfs/Practice%20Management/ASCCP_Cervical_Cancer_Screening_Recommendations.pdf#zoom=80. Accessed July 6, 2013.
29. American Cancer Society. New screening guidelines for cervical cancer. March 14, 2012. www.cancer.org/cancer/news/news/new-screening-guidelines-for-cervical-cancer. Accessed July 23, 2013.
30. American Congress of Obstetricians and Gynecologists. USPSTF updated cervical cancer screening. www.acog.org/~/media/Districts/District%20II/PDFs/USPSTF_Cervical_Ca_Screening_Guidelines.pdf?dmc=1&ts=20130723T1510069242. Accessed July 23, 2013.
31. CDC. Cervical cancer screening guidelines for average-risk women. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed July 5, 2013.
32. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880-891.
33. US Preventive Services Task Force. Screening for cervical cancer. Current recommendation. www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm#clinical. Accessed July 6, 2013.
34. American College of Obstetricians and Gynecologists; Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120:1222-1238.
35. Warman J. Cervical cancer screening in young women: saving lives with prevention and detection. Oncol Nurs Forum. 2010;37:33-38.
36. Gardasil [package insert]. Whitehouse Station, NJ: Merck & Co; 2011.
37. Cervarix [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
38. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
39. Morrison RS, Moody R, Shelton M. Pap smear rates: predictor of cervical cancer mortality disparity? Online J Rural Nurs Health Care. 2010;10:21-27.
40. National Cancer Institute. Cervical cancer treatment. www.cancer.gov/cancertopics/pdq/treatment/cervical/HealthProfessional/page1/AllPages#2.
41. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-104.
42. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99. management of abnormal cervical cytology and histology. J Obstet Gynecol. 2008;112:1419-1444.
43. Bowring J, Tulloch I, Phadnis SV, et al. Secondary excision for cervical intraepithelial neoplasia: an evaluation of two treatment methods. J Obstet Gynaecol. 2010;30:511-514.
44. Jhingran A. Neoplasms of the cervix. In: Hong KW, Bast RC Jr, Hait WN, et al, eds. Holland-Frei Cancer Medicine. 8th ed. Ontario, Canada: BC Decker; 2010:1304-1312.
45. Kim SH. Preinvasive disease of the lower genital tract. In: Chu CS, Rubin SC, eds. Manual of Gynecologic Oncology. 1st ed. Hackensack, NJ: World Scientific Publishing Company; 2011:80-87.
46. American Cancer Society. Cervical cancer key statistics. http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statistics. Accessed July 5, 2013.