User login
Endometriosis: From Identification to Management
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
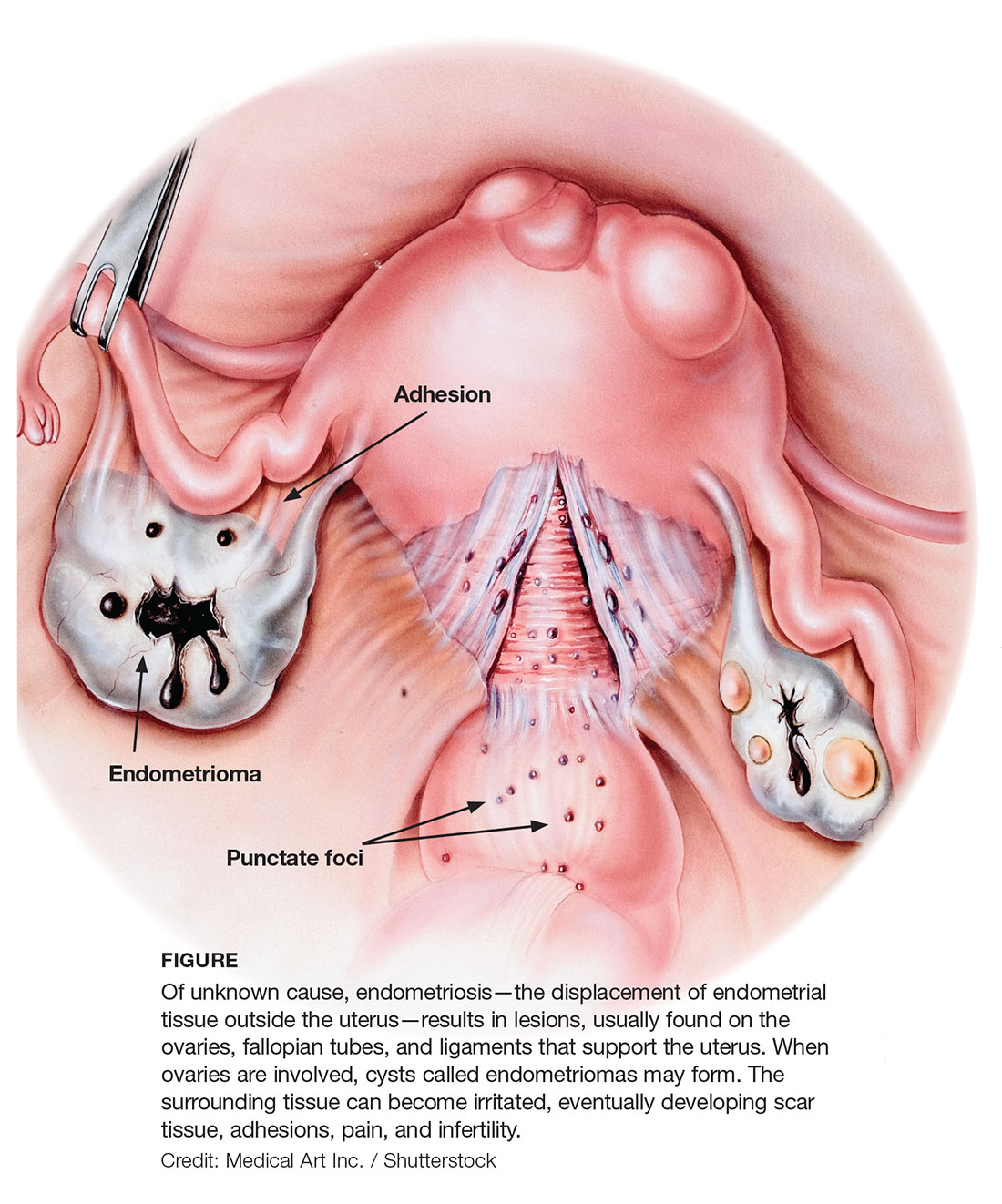
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
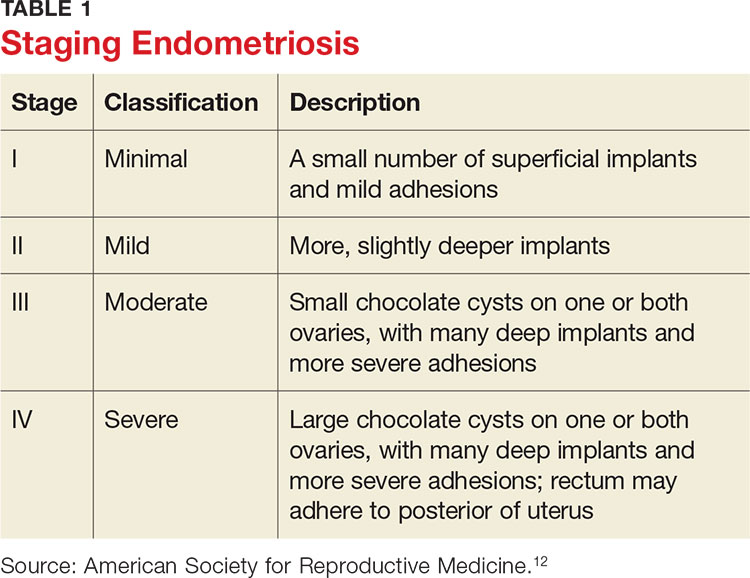
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
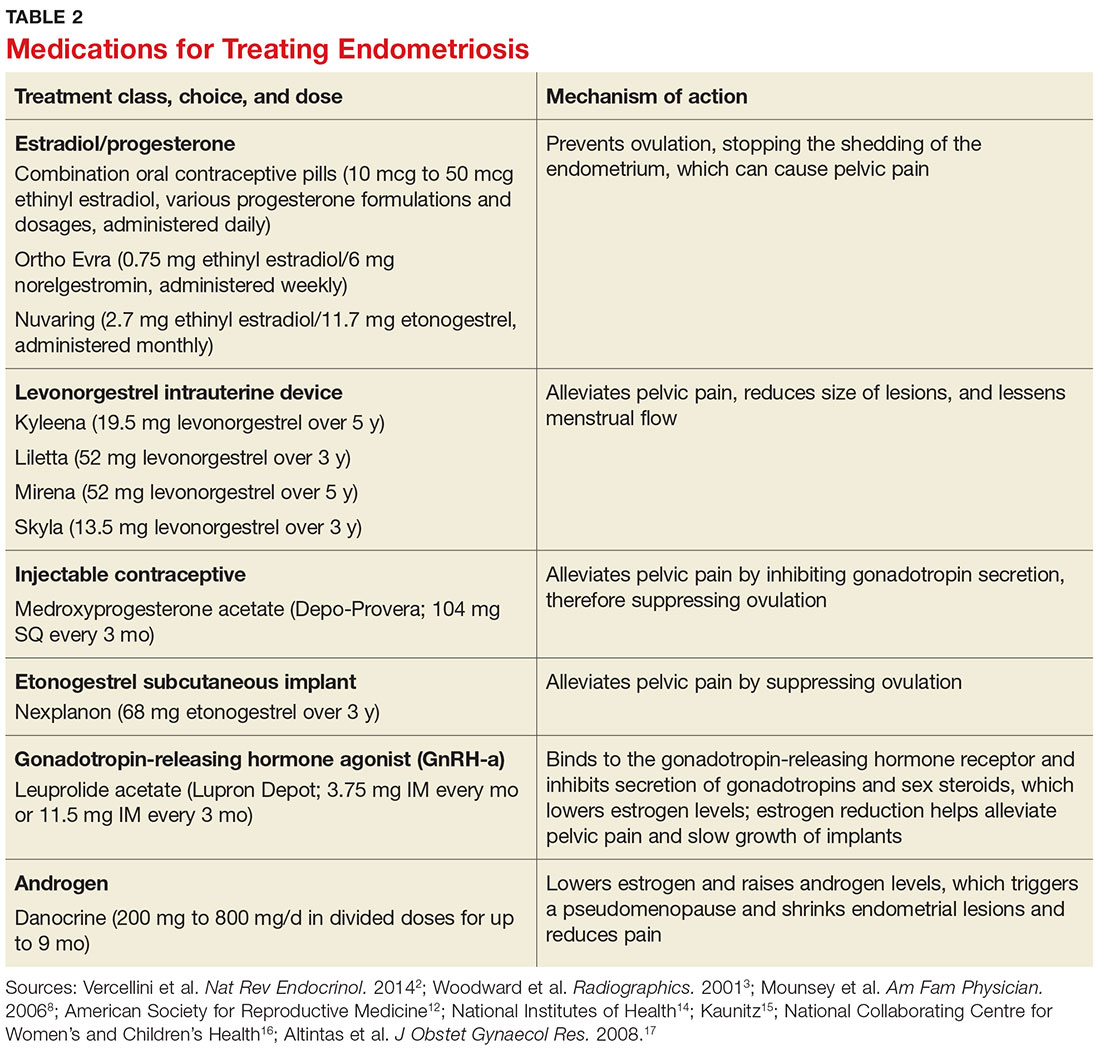
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.