User login
Primary Mucinous Carcinoma of the Skin
To the Editor:
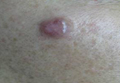
A 41-year-old man presented to our dermatology clinic with a recurrent asymptomatic nodule on the right cheek that had gradually increased in size over 1 year. The patient underwent laser excision at an outside facility 1 year after the first presentation. Pathology reports and tissue cultures from the excision were not available. The lesion recurred in the same location 6 months following excision. The patient reported no history of pain, fever, cough, weight loss, or loss of appetite, and he denied any trauma or radiation to the affected area. Dermatologic examination revealed a 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek (Figure 1). There was no tenderness or pruritus. Clinical examination and extensive radiographic studies revealed no primary disease. A complete blood cell count, biochemical tests, and serous tumor markers were within reference range. The lesion was resected with a 2-mm margin. The margins were free of tumor cells. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa and scattered floating islands of tumor cells in the dermis. Small-sized glands were organized in some areas of the tumor cells (Figure 2). Epithelial tumor cell islands contained uniform oval nuclei and focally vacuolated cytoplasm. The tumor cells were positive for cytokeratin 7 (CK7) (Figure 3) and negative for cytokeratin 20 (CK20), carcinoembryonic antigen, homeobox transcription factor (CDX2), villin, mucus-associated peptides of the thyroid transcription factor (TTF1), and prostate-specific antigen, all indicating a diagnosis of primary mucinous carcinoma of the skin (PMCS). No local recurrence, regional lymph node involvement, or distant metastasis was observed after resection.
 |
| Figure 1. A 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek. |
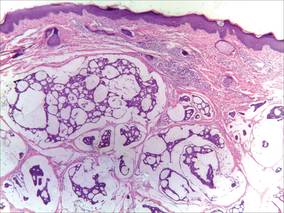 |
| Figure 2. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa that contained scattered floating islands of tumor cells in the dermis (H&E, original magnification ×40). |
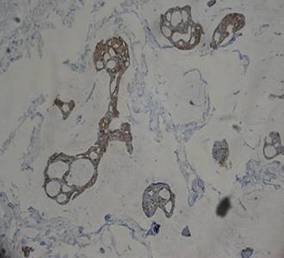 |
| Figure 3. The tumor cells were positive for cytokeratin 7 (original magnification ×400). |
Primary mucinous carcinoma of the skin is a rare malignant adnexal tumor. It was first described by Lennox et al1 in 1952 and was later given its name by Mendoza and Helwig2 through summarizing clinical and histochemical characteristics based on the observation of 14 cases. A PubMed search of articles indexed for MEDLINE using the search terms adenocarcinoma, mucinous (Medical Subject Headings); mucinous adenocarcinoma (title/abstract); colloid carcinoma* (title/abstract); skin neoplasms (Medical Subject Headings); skin neoplasm (title/abstract); skin cancer* (title/abstract), and primary (title/abstract) revealed more than 200 reported cases of PMCS. It usually is located in the head and neck region, with the eyelids being the most common site of presentation, but rare presentations in the gluteal region, axillae, hemithoraxes, vulva, arms, and legs also have been reported.3-6 Primary mucinous carcinoma of the skin mainly affects elderly patients (age range, 61–80 years), with men being affected more often than women. It often presents as a painless, solitary, nontender, sometimes ulcerated, translucent, white or reddish nodule. The tumors generally grow slowly with high rates of local recurrence and rare chances of distant metastasis.7 Most metastases involve the regional lymph nodes and lungs, but rare cases of bone marrow and parotid gland metastases also have been reported.8 Because PMCS is an indolent tumor, which may be mistaken for a benign tumor, it is not always histologically examined on initial presentation.
Primary mucinous carcinoma of the skin is characterized histopathologically by floating epithelial cell nests in mucinous lakes. It is unknown if this neoplasm shows eccrine- or apocrine-type differentiation. S-100 protein seems to indicate eccrine rather than apocrine differentiation.9 Misdiagnosis of PMCS is common, as it has an uncharacteristic gross appearance and may microscopically resemble cutaneous metastasis from a mucinous carcinoma of the breast, gastrointestinal tract, lungs, ovaries, or prostate. It is important to distinguish between metastatic tumors and PMCS because PMCS generally is more benign and has a better prognosis. A variety of stains have been reported as helpful in the diagnosis of PMCS, including CK7 and CK20, D2-40, p63, and others. In some reports, labeling for CK7 and CK20 was helpful in differentiating colon carcinoma from breast or skin malignancies but did not differentiate primary skin lesions from metastasis of breast malignancies to the skin.10-13 Paradela et al5 proposed D2-40 as a reliable immunostain to detect lymphatic invasion in node-negative primary tumors. Kalebi and Hale11 and Ivan et al12 highlighted the importance of p63 in excluding metastasis to the skin, particularly from the gastrointestinal tract, pancreaticobiliary tree, and lungs.
Even though these factors may help in establishing the primary site of the tumor, a final diagnosis only can be drawn when the patient has been subjected to a thorough investigation. In our patient, clinical workup did not reveal other possible primary sites in other organs or evidence of metastasis from the skin lesion to other sites. Based on the histologic findings, the diagnosis of PMCS was made.
The recommended treatment of PMCS varies from standard excision to wide local excision including dissection of the regional lymph nodes. Alam et al14 reported that Mohs micrographic surgery may be appropriate for first-line therapy because the procedure gives complete margin control and spares tissue. Immunohistochemistry should be used as an adjunct to routine hematoxylin and eosin staining to aid in ensuring negative margins.15 Considering a high potential for recurrence following surgical excision, it is important to detect hormone receptors in this tumor because patients may be treated using antiestrogenic drugs such as tamoxifen. Moreover, recurrent or metastatic PMCS is both resistant to radiotherapy and unresponsive to chemotherapy.3 Annual follow-up is recommended due to the potential for recurrence or metastasis.
1. Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin, with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-890.
2. Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
3. Breiting L, Dahlstrøm K, Christensen L, et al. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2007;29:595-596.
4. Krishnamurthy J, Saba F, Sunila. Primary mucinous carcinoma of the skin: a rare tumor in the gluteal region. Indian J Pathol Microbiol. 2009;52:225-227.
5. Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
6. Laco J, Simáková E, Svobodová J, et al. Recurrent mucinous carcinoma of skin mimicking primary mucinous carcinoma of parotid gland: a diagnostic pitfall. Cesk Patol. 2009;45:79-82.
7. Breiting L, Christensen L, Dahlstrøm K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
8. Ajithkumar TV, Nileena N, Abraham EK, et al. Bone marrow relapse in primary mucinous carcinoma of skin. Am J Clin Oncol. 1999;22:303-304.
9. Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
10. Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin [published online ahead of print September 24, 2009]. J Cutan Pathol. 2010;37:411-415.
11. Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
12. Ivan D, Nash JW, Prieto VG, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2007;34:474-480.
13. Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
14. Alam M, Trela R, Kim N, et al. Treatment of primary mucinous carcinoma of the skin: meta-analysis of 189 cases. J Invest Dermatol. 2009;129:382.
15. Stranahan D, Cherpelis BS, Glass LF, et al. Immunohistochemical stains in Mohs surgery: a review [published online ahead of print April 16, 2009]. Dermatol Surg. 2009;35:1023-1034.
To the Editor:
A 41-year-old man presented to our dermatology clinic with a recurrent asymptomatic nodule on the right cheek that had gradually increased in size over 1 year. The patient underwent laser excision at an outside facility 1 year after the first presentation. Pathology reports and tissue cultures from the excision were not available. The lesion recurred in the same location 6 months following excision. The patient reported no history of pain, fever, cough, weight loss, or loss of appetite, and he denied any trauma or radiation to the affected area. Dermatologic examination revealed a 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek (Figure 1). There was no tenderness or pruritus. Clinical examination and extensive radiographic studies revealed no primary disease. A complete blood cell count, biochemical tests, and serous tumor markers were within reference range. The lesion was resected with a 2-mm margin. The margins were free of tumor cells. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa and scattered floating islands of tumor cells in the dermis. Small-sized glands were organized in some areas of the tumor cells (Figure 2). Epithelial tumor cell islands contained uniform oval nuclei and focally vacuolated cytoplasm. The tumor cells were positive for cytokeratin 7 (CK7) (Figure 3) and negative for cytokeratin 20 (CK20), carcinoembryonic antigen, homeobox transcription factor (CDX2), villin, mucus-associated peptides of the thyroid transcription factor (TTF1), and prostate-specific antigen, all indicating a diagnosis of primary mucinous carcinoma of the skin (PMCS). No local recurrence, regional lymph node involvement, or distant metastasis was observed after resection.
 |
| Figure 1. A 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek. |
 |
| Figure 2. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa that contained scattered floating islands of tumor cells in the dermis (H&E, original magnification ×40). |
 |
| Figure 3. The tumor cells were positive for cytokeratin 7 (original magnification ×400). |
Primary mucinous carcinoma of the skin is a rare malignant adnexal tumor. It was first described by Lennox et al1 in 1952 and was later given its name by Mendoza and Helwig2 through summarizing clinical and histochemical characteristics based on the observation of 14 cases. A PubMed search of articles indexed for MEDLINE using the search terms adenocarcinoma, mucinous (Medical Subject Headings); mucinous adenocarcinoma (title/abstract); colloid carcinoma* (title/abstract); skin neoplasms (Medical Subject Headings); skin neoplasm (title/abstract); skin cancer* (title/abstract), and primary (title/abstract) revealed more than 200 reported cases of PMCS. It usually is located in the head and neck region, with the eyelids being the most common site of presentation, but rare presentations in the gluteal region, axillae, hemithoraxes, vulva, arms, and legs also have been reported.3-6 Primary mucinous carcinoma of the skin mainly affects elderly patients (age range, 61–80 years), with men being affected more often than women. It often presents as a painless, solitary, nontender, sometimes ulcerated, translucent, white or reddish nodule. The tumors generally grow slowly with high rates of local recurrence and rare chances of distant metastasis.7 Most metastases involve the regional lymph nodes and lungs, but rare cases of bone marrow and parotid gland metastases also have been reported.8 Because PMCS is an indolent tumor, which may be mistaken for a benign tumor, it is not always histologically examined on initial presentation.
Primary mucinous carcinoma of the skin is characterized histopathologically by floating epithelial cell nests in mucinous lakes. It is unknown if this neoplasm shows eccrine- or apocrine-type differentiation. S-100 protein seems to indicate eccrine rather than apocrine differentiation.9 Misdiagnosis of PMCS is common, as it has an uncharacteristic gross appearance and may microscopically resemble cutaneous metastasis from a mucinous carcinoma of the breast, gastrointestinal tract, lungs, ovaries, or prostate. It is important to distinguish between metastatic tumors and PMCS because PMCS generally is more benign and has a better prognosis. A variety of stains have been reported as helpful in the diagnosis of PMCS, including CK7 and CK20, D2-40, p63, and others. In some reports, labeling for CK7 and CK20 was helpful in differentiating colon carcinoma from breast or skin malignancies but did not differentiate primary skin lesions from metastasis of breast malignancies to the skin.10-13 Paradela et al5 proposed D2-40 as a reliable immunostain to detect lymphatic invasion in node-negative primary tumors. Kalebi and Hale11 and Ivan et al12 highlighted the importance of p63 in excluding metastasis to the skin, particularly from the gastrointestinal tract, pancreaticobiliary tree, and lungs.
Even though these factors may help in establishing the primary site of the tumor, a final diagnosis only can be drawn when the patient has been subjected to a thorough investigation. In our patient, clinical workup did not reveal other possible primary sites in other organs or evidence of metastasis from the skin lesion to other sites. Based on the histologic findings, the diagnosis of PMCS was made.
The recommended treatment of PMCS varies from standard excision to wide local excision including dissection of the regional lymph nodes. Alam et al14 reported that Mohs micrographic surgery may be appropriate for first-line therapy because the procedure gives complete margin control and spares tissue. Immunohistochemistry should be used as an adjunct to routine hematoxylin and eosin staining to aid in ensuring negative margins.15 Considering a high potential for recurrence following surgical excision, it is important to detect hormone receptors in this tumor because patients may be treated using antiestrogenic drugs such as tamoxifen. Moreover, recurrent or metastatic PMCS is both resistant to radiotherapy and unresponsive to chemotherapy.3 Annual follow-up is recommended due to the potential for recurrence or metastasis.
To the Editor:
A 41-year-old man presented to our dermatology clinic with a recurrent asymptomatic nodule on the right cheek that had gradually increased in size over 1 year. The patient underwent laser excision at an outside facility 1 year after the first presentation. Pathology reports and tissue cultures from the excision were not available. The lesion recurred in the same location 6 months following excision. The patient reported no history of pain, fever, cough, weight loss, or loss of appetite, and he denied any trauma or radiation to the affected area. Dermatologic examination revealed a 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek (Figure 1). There was no tenderness or pruritus. Clinical examination and extensive radiographic studies revealed no primary disease. A complete blood cell count, biochemical tests, and serous tumor markers were within reference range. The lesion was resected with a 2-mm margin. The margins were free of tumor cells. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa and scattered floating islands of tumor cells in the dermis. Small-sized glands were organized in some areas of the tumor cells (Figure 2). Epithelial tumor cell islands contained uniform oval nuclei and focally vacuolated cytoplasm. The tumor cells were positive for cytokeratin 7 (CK7) (Figure 3) and negative for cytokeratin 20 (CK20), carcinoembryonic antigen, homeobox transcription factor (CDX2), villin, mucus-associated peptides of the thyroid transcription factor (TTF1), and prostate-specific antigen, all indicating a diagnosis of primary mucinous carcinoma of the skin (PMCS). No local recurrence, regional lymph node involvement, or distant metastasis was observed after resection.
 |
| Figure 1. A 24×18-mm, slightly elevated, dome-shaped, translucent, pink-colored tumor with telangiectasia on the right cheek. |
 |
| Figure 2. Histopathology showed a circumscribed tumor with large amounts of mucin compartmentalized by fibrous septa that contained scattered floating islands of tumor cells in the dermis (H&E, original magnification ×40). |
 |
| Figure 3. The tumor cells were positive for cytokeratin 7 (original magnification ×400). |
Primary mucinous carcinoma of the skin is a rare malignant adnexal tumor. It was first described by Lennox et al1 in 1952 and was later given its name by Mendoza and Helwig2 through summarizing clinical and histochemical characteristics based on the observation of 14 cases. A PubMed search of articles indexed for MEDLINE using the search terms adenocarcinoma, mucinous (Medical Subject Headings); mucinous adenocarcinoma (title/abstract); colloid carcinoma* (title/abstract); skin neoplasms (Medical Subject Headings); skin neoplasm (title/abstract); skin cancer* (title/abstract), and primary (title/abstract) revealed more than 200 reported cases of PMCS. It usually is located in the head and neck region, with the eyelids being the most common site of presentation, but rare presentations in the gluteal region, axillae, hemithoraxes, vulva, arms, and legs also have been reported.3-6 Primary mucinous carcinoma of the skin mainly affects elderly patients (age range, 61–80 years), with men being affected more often than women. It often presents as a painless, solitary, nontender, sometimes ulcerated, translucent, white or reddish nodule. The tumors generally grow slowly with high rates of local recurrence and rare chances of distant metastasis.7 Most metastases involve the regional lymph nodes and lungs, but rare cases of bone marrow and parotid gland metastases also have been reported.8 Because PMCS is an indolent tumor, which may be mistaken for a benign tumor, it is not always histologically examined on initial presentation.
Primary mucinous carcinoma of the skin is characterized histopathologically by floating epithelial cell nests in mucinous lakes. It is unknown if this neoplasm shows eccrine- or apocrine-type differentiation. S-100 protein seems to indicate eccrine rather than apocrine differentiation.9 Misdiagnosis of PMCS is common, as it has an uncharacteristic gross appearance and may microscopically resemble cutaneous metastasis from a mucinous carcinoma of the breast, gastrointestinal tract, lungs, ovaries, or prostate. It is important to distinguish between metastatic tumors and PMCS because PMCS generally is more benign and has a better prognosis. A variety of stains have been reported as helpful in the diagnosis of PMCS, including CK7 and CK20, D2-40, p63, and others. In some reports, labeling for CK7 and CK20 was helpful in differentiating colon carcinoma from breast or skin malignancies but did not differentiate primary skin lesions from metastasis of breast malignancies to the skin.10-13 Paradela et al5 proposed D2-40 as a reliable immunostain to detect lymphatic invasion in node-negative primary tumors. Kalebi and Hale11 and Ivan et al12 highlighted the importance of p63 in excluding metastasis to the skin, particularly from the gastrointestinal tract, pancreaticobiliary tree, and lungs.
Even though these factors may help in establishing the primary site of the tumor, a final diagnosis only can be drawn when the patient has been subjected to a thorough investigation. In our patient, clinical workup did not reveal other possible primary sites in other organs or evidence of metastasis from the skin lesion to other sites. Based on the histologic findings, the diagnosis of PMCS was made.
The recommended treatment of PMCS varies from standard excision to wide local excision including dissection of the regional lymph nodes. Alam et al14 reported that Mohs micrographic surgery may be appropriate for first-line therapy because the procedure gives complete margin control and spares tissue. Immunohistochemistry should be used as an adjunct to routine hematoxylin and eosin staining to aid in ensuring negative margins.15 Considering a high potential for recurrence following surgical excision, it is important to detect hormone receptors in this tumor because patients may be treated using antiestrogenic drugs such as tamoxifen. Moreover, recurrent or metastatic PMCS is both resistant to radiotherapy and unresponsive to chemotherapy.3 Annual follow-up is recommended due to the potential for recurrence or metastasis.
1. Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin, with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-890.
2. Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
3. Breiting L, Dahlstrøm K, Christensen L, et al. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2007;29:595-596.
4. Krishnamurthy J, Saba F, Sunila. Primary mucinous carcinoma of the skin: a rare tumor in the gluteal region. Indian J Pathol Microbiol. 2009;52:225-227.
5. Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
6. Laco J, Simáková E, Svobodová J, et al. Recurrent mucinous carcinoma of skin mimicking primary mucinous carcinoma of parotid gland: a diagnostic pitfall. Cesk Patol. 2009;45:79-82.
7. Breiting L, Christensen L, Dahlstrøm K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
8. Ajithkumar TV, Nileena N, Abraham EK, et al. Bone marrow relapse in primary mucinous carcinoma of skin. Am J Clin Oncol. 1999;22:303-304.
9. Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
10. Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin [published online ahead of print September 24, 2009]. J Cutan Pathol. 2010;37:411-415.
11. Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
12. Ivan D, Nash JW, Prieto VG, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2007;34:474-480.
13. Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
14. Alam M, Trela R, Kim N, et al. Treatment of primary mucinous carcinoma of the skin: meta-analysis of 189 cases. J Invest Dermatol. 2009;129:382.
15. Stranahan D, Cherpelis BS, Glass LF, et al. Immunohistochemical stains in Mohs surgery: a review [published online ahead of print April 16, 2009]. Dermatol Surg. 2009;35:1023-1034.
1. Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin, with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952;64:865-890.
2. Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971;103:68-78.
3. Breiting L, Dahlstrøm K, Christensen L, et al. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2007;29:595-596.
4. Krishnamurthy J, Saba F, Sunila. Primary mucinous carcinoma of the skin: a rare tumor in the gluteal region. Indian J Pathol Microbiol. 2009;52:225-227.
5. Paradela S, Castiñeiras I, Cuevas J, et al. Mucinous carcinoma of the skin: evaluation of lymphatic invasion with D2-40. Am J Dermatopathol. 2008;30:504-508.
6. Laco J, Simáková E, Svobodová J, et al. Recurrent mucinous carcinoma of skin mimicking primary mucinous carcinoma of parotid gland: a diagnostic pitfall. Cesk Patol. 2009;45:79-82.
7. Breiting L, Christensen L, Dahlstrøm K, et al. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242-245.
8. Ajithkumar TV, Nileena N, Abraham EK, et al. Bone marrow relapse in primary mucinous carcinoma of skin. Am J Clin Oncol. 1999;22:303-304.
9. Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000;22:166-170.
10. Levy G, Finkelstein A, McNiff JM. Immunohistochemical techniques to compare primary vs. metastatic mucinous carcinoma of the skin [published online ahead of print September 24, 2009]. J Cutan Pathol. 2010;37:411-415.
11. Kalebi A, Hale M. Primary mucinous carcinoma of the skin: usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol. 2008;30:510.
12. Ivan D, Nash JW, Prieto VG, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2007;34:474-480.
13. Papalas JA, Proia AD. Primary mucinous carcinoma of the eyelid: a clinicopathologic and immunohistochemical study of 4 cases and an update on recurrence rates. Arch Ophthalmol. 2010;128:1160-1165.
14. Alam M, Trela R, Kim N, et al. Treatment of primary mucinous carcinoma of the skin: meta-analysis of 189 cases. J Invest Dermatol. 2009;129:382.
15. Stranahan D, Cherpelis BS, Glass LF, et al. Immunohistochemical stains in Mohs surgery: a review [published online ahead of print April 16, 2009]. Dermatol Surg. 2009;35:1023-1034.