User login
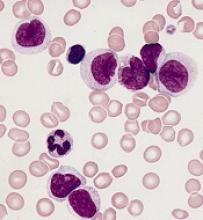
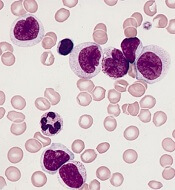
CHMP reconsiders new indication for blinatumomab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
CHMP backs proposed biosimilars of pegfilgrastim
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
CHMP recommends factor VIII therapy for hemophilia A
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
CHMP supports new indication for venetoclax
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
CHMP recommends mogamulizumab for MF, SS
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
NGS can predict AML relapse after HSCT
Next-generation sequencing (NGS) can be used to predict relapse in acute myeloid leukemia (AML) patients undergoing hematopoietic stem cell transplant (HSCT), according to research published in Blood.
Researchers found that patients with a higher variant allele frequency (VAF) 21 days after HSCT had a higher risk of relapse and death.
“We can detect mutations in patients’ bone marrow cells 3 weeks after the transplant and, based on that, predict the likelihood of their relapse,” explained study author Zhaolei Zhang, PhD, of the University of Toronto in Ontario, Canada.
Dr. Zhang and his colleagues performed NGS on 529 bone marrow samples from 104 AML patients who underwent chemotherapy and HSCT.
The samples were collected at the time of diagnosis, during the chemotherapy-induced remission, and 3 weeks after HSCT. A subset of patients also gave samples at 3 months, 6 months, and 12 months after HSCT.
The researchers identified 256 mutations that were present in 90 patients at diagnosis and looked for those same mutations at each sampling point.
Chemotherapy and HSCT eliminated most AML cells, leading to a reduction in mutation frequency. However, in some patients, mutations observed at diagnosis could still be detected after chemotherapy and at day 21 after HSCT, indicating the presence of treatment-resistant AML cells.
Allelic burdens at day 21 post-HSCT were higher in patients who ultimately relapsed, and the mutations observed at day 21 expanded at relapse.
The 3-year relapse rate was 56.2% in patients with a VAF greater than 0.2% at day 21 post-HSCT, compared to 16.0% in patients with a lower or no mutational burden (P<0.001).
The 3-year overall survival rates were 36.5% in patients with a VAF greater than 0.2% and 67.0% for patients with a lower or no mutational burden (P=0.006).
In multivariate analyses, VAF0.2% at day 21 was an independent adverse prognostic factor for relapse—hazard ratio, 4.75 (P<0.001)—and overall survival—hazard ratio, 3.07 (P=0.003).
Dr. Zhang and his colleagues said these results suggest NGS-based monitoring after HSCT “provides valuable information” that, together with a patient’s baseline mutational profile and clinical evaluation, can be used to predict outcomes of transplant.
This study was supported by research grants from the Natural Science and Engineering Council of Canada, Leukemia and Lymphoma Society of Canada, Princess Margaret Foundation, National Research Foundation of Korea, and National Natural Science Foundation of China.
Next-generation sequencing (NGS) can be used to predict relapse in acute myeloid leukemia (AML) patients undergoing hematopoietic stem cell transplant (HSCT), according to research published in Blood.
Researchers found that patients with a higher variant allele frequency (VAF) 21 days after HSCT had a higher risk of relapse and death.
“We can detect mutations in patients’ bone marrow cells 3 weeks after the transplant and, based on that, predict the likelihood of their relapse,” explained study author Zhaolei Zhang, PhD, of the University of Toronto in Ontario, Canada.
Dr. Zhang and his colleagues performed NGS on 529 bone marrow samples from 104 AML patients who underwent chemotherapy and HSCT.
The samples were collected at the time of diagnosis, during the chemotherapy-induced remission, and 3 weeks after HSCT. A subset of patients also gave samples at 3 months, 6 months, and 12 months after HSCT.
The researchers identified 256 mutations that were present in 90 patients at diagnosis and looked for those same mutations at each sampling point.
Chemotherapy and HSCT eliminated most AML cells, leading to a reduction in mutation frequency. However, in some patients, mutations observed at diagnosis could still be detected after chemotherapy and at day 21 after HSCT, indicating the presence of treatment-resistant AML cells.
Allelic burdens at day 21 post-HSCT were higher in patients who ultimately relapsed, and the mutations observed at day 21 expanded at relapse.
The 3-year relapse rate was 56.2% in patients with a VAF greater than 0.2% at day 21 post-HSCT, compared to 16.0% in patients with a lower or no mutational burden (P<0.001).
The 3-year overall survival rates were 36.5% in patients with a VAF greater than 0.2% and 67.0% for patients with a lower or no mutational burden (P=0.006).
In multivariate analyses, VAF0.2% at day 21 was an independent adverse prognostic factor for relapse—hazard ratio, 4.75 (P<0.001)—and overall survival—hazard ratio, 3.07 (P=0.003).
Dr. Zhang and his colleagues said these results suggest NGS-based monitoring after HSCT “provides valuable information” that, together with a patient’s baseline mutational profile and clinical evaluation, can be used to predict outcomes of transplant.
This study was supported by research grants from the Natural Science and Engineering Council of Canada, Leukemia and Lymphoma Society of Canada, Princess Margaret Foundation, National Research Foundation of Korea, and National Natural Science Foundation of China.
Next-generation sequencing (NGS) can be used to predict relapse in acute myeloid leukemia (AML) patients undergoing hematopoietic stem cell transplant (HSCT), according to research published in Blood.
Researchers found that patients with a higher variant allele frequency (VAF) 21 days after HSCT had a higher risk of relapse and death.
“We can detect mutations in patients’ bone marrow cells 3 weeks after the transplant and, based on that, predict the likelihood of their relapse,” explained study author Zhaolei Zhang, PhD, of the University of Toronto in Ontario, Canada.
Dr. Zhang and his colleagues performed NGS on 529 bone marrow samples from 104 AML patients who underwent chemotherapy and HSCT.
The samples were collected at the time of diagnosis, during the chemotherapy-induced remission, and 3 weeks after HSCT. A subset of patients also gave samples at 3 months, 6 months, and 12 months after HSCT.
The researchers identified 256 mutations that were present in 90 patients at diagnosis and looked for those same mutations at each sampling point.
Chemotherapy and HSCT eliminated most AML cells, leading to a reduction in mutation frequency. However, in some patients, mutations observed at diagnosis could still be detected after chemotherapy and at day 21 after HSCT, indicating the presence of treatment-resistant AML cells.
Allelic burdens at day 21 post-HSCT were higher in patients who ultimately relapsed, and the mutations observed at day 21 expanded at relapse.
The 3-year relapse rate was 56.2% in patients with a VAF greater than 0.2% at day 21 post-HSCT, compared to 16.0% in patients with a lower or no mutational burden (P<0.001).
The 3-year overall survival rates were 36.5% in patients with a VAF greater than 0.2% and 67.0% for patients with a lower or no mutational burden (P=0.006).
In multivariate analyses, VAF0.2% at day 21 was an independent adverse prognostic factor for relapse—hazard ratio, 4.75 (P<0.001)—and overall survival—hazard ratio, 3.07 (P=0.003).
Dr. Zhang and his colleagues said these results suggest NGS-based monitoring after HSCT “provides valuable information” that, together with a patient’s baseline mutational profile and clinical evaluation, can be used to predict outcomes of transplant.
This study was supported by research grants from the Natural Science and Engineering Council of Canada, Leukemia and Lymphoma Society of Canada, Princess Margaret Foundation, National Research Foundation of Korea, and National Natural Science Foundation of China.
Trial sponsors fail to comply with EU rules
Sponsors of clinical trials often do not comply with European Union (EU) rules on reporting results, according to a new study.
Researchers looked at more than 7,000 trials for which results were supposed to be reported on the EU Clinical Trials Register (EUCTR).
Only about half of these trials actually had results on the EUCTR within 12 months of trial completion, as is required by the European Commission.
Ben Goldacre, MBBS, of the University of Oxford in the U.K., and his colleagues reported these findings in The BMJ.
The researchers assessed compliance with EU rules, explored factors associated with non-compliance, and ranked sponsors by compliance. The team also created a website for live, ongoing audit of compliance.
The researchers assessed 7,274 completed clinical trials for which results were due and found that 49.5% had results reported on the EUCTR.
Trials with commercial sponsors were more likely to have posted results than trials with non-commercial sponsors—68.1% and 11.0%, respectively (adjusted odds ratio, 23.25; P<0.001).
In addition, sponsors with a large number of trials were more likely to report results than sponsors without a large number of trials—78% and 18%, respectively (adjusted odds ratio, 18.38; P<0.001).
The researchers also said they found evidence of errors, omissions, and contradictory data on the EUCTR. For example, 29.4% of trials marked as “completed” gave no completion date, which prevents ascertainment of compliance with reporting requirements.
Finally, the researchers described the development of the EU TrialsTracker website. This site gives detailed information on the trial reporting performance of every individual drug company, university, and hospital conducting clinical trials in Europe.
The site shows which sponsors are the best or worst at complying with the law, and it gives detailed information on the individual trials for which results have not been reported on the register. The information on the site is updated every month.
Sponsors of clinical trials often do not comply with European Union (EU) rules on reporting results, according to a new study.
Researchers looked at more than 7,000 trials for which results were supposed to be reported on the EU Clinical Trials Register (EUCTR).
Only about half of these trials actually had results on the EUCTR within 12 months of trial completion, as is required by the European Commission.
Ben Goldacre, MBBS, of the University of Oxford in the U.K., and his colleagues reported these findings in The BMJ.
The researchers assessed compliance with EU rules, explored factors associated with non-compliance, and ranked sponsors by compliance. The team also created a website for live, ongoing audit of compliance.
The researchers assessed 7,274 completed clinical trials for which results were due and found that 49.5% had results reported on the EUCTR.
Trials with commercial sponsors were more likely to have posted results than trials with non-commercial sponsors—68.1% and 11.0%, respectively (adjusted odds ratio, 23.25; P<0.001).
In addition, sponsors with a large number of trials were more likely to report results than sponsors without a large number of trials—78% and 18%, respectively (adjusted odds ratio, 18.38; P<0.001).
The researchers also said they found evidence of errors, omissions, and contradictory data on the EUCTR. For example, 29.4% of trials marked as “completed” gave no completion date, which prevents ascertainment of compliance with reporting requirements.
Finally, the researchers described the development of the EU TrialsTracker website. This site gives detailed information on the trial reporting performance of every individual drug company, university, and hospital conducting clinical trials in Europe.
The site shows which sponsors are the best or worst at complying with the law, and it gives detailed information on the individual trials for which results have not been reported on the register. The information on the site is updated every month.
Sponsors of clinical trials often do not comply with European Union (EU) rules on reporting results, according to a new study.
Researchers looked at more than 7,000 trials for which results were supposed to be reported on the EU Clinical Trials Register (EUCTR).
Only about half of these trials actually had results on the EUCTR within 12 months of trial completion, as is required by the European Commission.
Ben Goldacre, MBBS, of the University of Oxford in the U.K., and his colleagues reported these findings in The BMJ.
The researchers assessed compliance with EU rules, explored factors associated with non-compliance, and ranked sponsors by compliance. The team also created a website for live, ongoing audit of compliance.
The researchers assessed 7,274 completed clinical trials for which results were due and found that 49.5% had results reported on the EUCTR.
Trials with commercial sponsors were more likely to have posted results than trials with non-commercial sponsors—68.1% and 11.0%, respectively (adjusted odds ratio, 23.25; P<0.001).
In addition, sponsors with a large number of trials were more likely to report results than sponsors without a large number of trials—78% and 18%, respectively (adjusted odds ratio, 18.38; P<0.001).
The researchers also said they found evidence of errors, omissions, and contradictory data on the EUCTR. For example, 29.4% of trials marked as “completed” gave no completion date, which prevents ascertainment of compliance with reporting requirements.
Finally, the researchers described the development of the EU TrialsTracker website. This site gives detailed information on the trial reporting performance of every individual drug company, university, and hospital conducting clinical trials in Europe.
The site shows which sponsors are the best or worst at complying with the law, and it gives detailed information on the individual trials for which results have not been reported on the register. The information on the site is updated every month.
Stress linked to disease markers in CLL
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
NICE rejects DLBCL indication for CAR T-cell therapy
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
OBI-3424 receives orphan designation for ALL
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.